Pediatric WBMRI in CPS: Case-Based Learning
1Division of Pediatric Radiology, Department of Radiology, University Hospital of Tübingen, Tuebingen, Germany
Synopsis
Approximately 10% of children and adolescents with cancer have an underlying CPS. Patients with CPS have an increased lifetime risk for a specific tumor. In addition to indications for imaging in cases of clinical suspicion, planned screenings to exclude clinical occult tumor manifestation are essential. Due to the increased sensitivity in CPS to ionizing radiation, MRI is favored. WB-MRI employs fluid-sensitive fat-suppressed 2D sequences as a baseline but must be supplemented with high-quality organ examination in cases of suspicious findings or high-risk constellations. With the latest techniques, such as compressed sensing, the very time-consuming measurements can be considerably shortened.
Pediatric WBMRI in CPS: Case-Based Learning
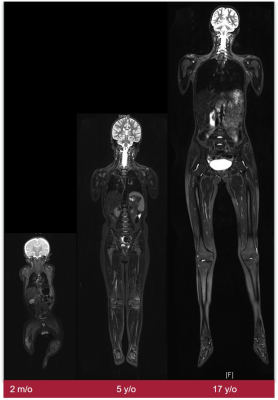
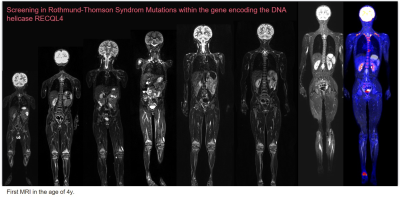
Approximately 10% of children and adolescents with cancer have an underlying cancer-predisposition syndrome (CPS). Common CPS include Li-Fraumeni syndrome, constitutional mismatch repair deficiency syndrome (CMMRD), hereditary paraganglioma, phaechromocytoma syndrome, rhabdoid tumor predisposition syndrome, hereditary retinoblastoma, and neurofibromatosis type1 (NF1). Patients with CPS have an increased lifetime risk of a specific tumor or of a typical spectrum of malignancies: for example, brain tumors, osteo- and soft-tissue sarcomas, adrenocortical carcinomas, and premenopausal breast cancer are more common in Li-Fraumeni syndrome. Children with hereditary retinoblastoma are more likely to develop osteosarcomas, soft tissue sarcomas, midface tumors, melanomas, as well as lung, breast, urinary bladder, uterine cancers. CMMRD is associated with brain tumors, gastrointestinal and hematologic malignancies. Typical benign manifestations in NF1 include peripheral nerve sheath tumors (PNST); malignant neoplasms include malignant PNST (MPNST), visual pathway gliomas, pilocytic astrocytomas, juvenile myelomonocytic leukemia, and, in middle age, breast cancer. In addition to indications for imaging in cases of clinical suspicion, planned screening to exclude clinical occult tumor manifestation is becoming increasingly important. Typical indications for imaging screening are asymptomatic children with a proven TDS, a family history of TDS-associated tumor, or children in whom clinical criteria with an increased risk profile suggest surveillance is appropriate. Because of the increased sensitivity in TDS to ionizing radiation, methods such as ultrasound and MRI are favored. The type and extent are essentially done by the risk profile including age and sex. Thus, patients with Li-Fraumeni syndrome have a nearly 100% cumulative risk of developing one or more cancers with disease peaking in childhood and in older adults. Because multifocality may involve other organ systems in addition to the common CNS tumors, a Whole-Body (WB) MRI examination including a diagnostic CNS MRI is recommended on an annual basis. In elderly patients who do not require sedation, an additional CNS MRI should be performed at 6-month intervals, if necessary. In addition, abdominal sonograms are performed at 3-4 monthly intervals. WBMRI uses fluid-sensitive fat-suppressed 2D sequences (STIR) and T1-weighted sequences. The combination of these two sequences in the context of screening requires a short examination time, but may need to be supplemented with a higher-quality organ examination in the presence of suspicious findings or high-risk constellations. Alternatively, a one-stage strategy can be pursued with special equipment. In this case, the high resolution of the WBMRI is aimed for and combined with a targeted examination (e.g., CNS) or with functional sequences. With the latest techniques, such as compressed sensing, the measurements, which may be very time-consuming, can be considerably shortened and thus also performed in awake children. Nevertheless, it is imperative to develop, harmonize, and evaluate in studies specific protocols for the different TDS with different risk constellations for the respective age groups.Acknowledgements
No acknowledgement found.References
Schäfer JF, Granata C, von Kalle T, et al. Whole-body magnetic resonance imaging in pediatric oncology - recommendations by the Oncology Task Force of the ESPR. Pediatric radiology. 2020; 50(8):1162-74
Greer MC. Imaging of cancer predisposition syndromes. Pediatric radiology. 2018; 48(9):1364-75.
Ballinger ML, Best A, Mai PL, et al. Baseline Surveillance in Li-Fraumeni Syndrome Using Whole-Body Magnetic Resonance Imaging: A Meta-analysis. JAMA oncology. 2017; 3(12):1634-9.