CS & AI: Will They Tread the Same Path to Translation?
R. Marc Lebel1
1GE Healthcare, Calgary, AB, Canada, Canada
1GE Healthcare, Calgary, AB, Canada, Canada
Synopsis
This course covers aspects related to translation and commercialization of new MR techniques. We will identify and explain factors paving the perilous road to commercial success with a specific focus on AI applications. The aim is to provide insight into the common reasons why novel applications falter and to provide design principles that improve chances of successful translation from bench to bedside.
Target Audience
Members of the MR community who would like to see broader adoption and clinical translation of the methods they are developing, implementing, and evaluating.Objectives
Audience members will gain an appreciation of factors that hinder translation and commercialization of novel techniques. Understanding these factors will enable participants to design applications that are effective, robust, and have a better chance of clinical translation.Introduction
Welcome to the ISMRM: the birthplace and deathbed of innovation in MRI! Every year the ISMRM accepts thousands of abstracts detailing ground-breaking innovations and revolutionary clinical breakthroughs. A minority of these methods percolate into commercial products with clinical indications for use or evolve into valuable research tools. Unfortunately, many innovations make little or no lasting impact and languish in perpetuity in the ISMRM archives. The resurgence of AI – often characterized by enigmatically impressive results – is likely exacerbating, not abating, this disparity. All abstracts presented at the ISMRM or published in respected journals undergo peer review and therefore provide convincing evidence of technical or clinical value so why isn’t there a higher translation rate?This educational course touches on topics that impact translation beyond those prioritized in academic journals and conference proceedings. Consideration of these factors when developing and testing new applications, especially those involving AI, should smooth the path to translation.
Academic versus commercial success
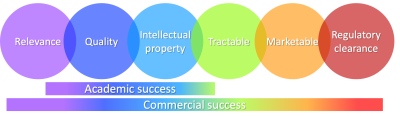
Once published and presented, all abstracts and papers have achieved a degree of academic success: a hypothesis was defined, experiments performed to support (or disprove) the hypothesis, and the results communicated. In MR, this may take the form of a clinical need, a new acquisition or algorithm, and a conference proceeding and/or journal article characterizing the solution in the context of the problem. The inventors and institution may file a patent. While academically successful, this approach requires additional work prior to successful translation. There are multiple considerations required for academic and commercial success, Figure 1. While there is considerable overlap in core areas there are some completely unique considerations required for translation. Furthermore, the areas that overlap often have different success criteria. In the following sections we will investigate these individual factors.Relevance
Techniques that translate successfully typically solve an unmet need. In MRI, this often takes the form of a clinical problem motivating a solution. This is not always the case in academia where basic research may precede a known application. Furthermore, academic applications may appeal to small, sub-specialized groups. A novel technique may not (yet) have sufficient relevance or appeal to enough users to pursue translation.Quality
Nearly every submission to the ISMRM presents results that appear sufficient for commercial translation. Techniques don’t fail because the best image is insufficient. They succeed because the worst image is acceptable. Showcasing the best results is an academically successful (and admittedly exciting) strategy but poses a serious challenge to separating the wheat from the chaff.Translation requires that a technique reliably solves a problem. A technique may perform perfectly on a small sample in a controlled environment but fail frequently in the field. Successful translation requires a very low failure rate. Corner cases may occur infrequently but when they occur, they are still valid cases and may have life changing implications to the patient. Failure rates and failure modes are central to successful translation.
Solution quality is of particular importance in AI techniques. These methods have the potential to be massively successful when applied to in-distribution inputs (data that is statistically identical to the training data) yet fail catastrophically to out-of-distribution inputs (data that is even slightly different than the training data). It is amazingly easy to prototype an AI technique that gives state-of-the-art results, sometimes with small quantities of training data. It is also common to train an AI algorithm to succeed at a desired task and to quantify success as the ability to achieve this task on similar data. Robust AI development should also focus on preventing and managing failures. Additionally, it should consider how the network achieves its task. Training AI algorithms can be very robust but only if specifically designed this way.
Intellectual property
Patents stimulate innovation by granting those who have invested resources in R&D the exclusive opportunity to profit. In practice, patents prevent others from implementing the specific innovations claimed in the patent. Unless the inventor/assignee brings the innovation to market, patents are a barrier to translation. The potential commercial success needs to justify licensing costs. Patents are a double-edged sword: incredibly valuable when a technique is highly effective but may inadvertently ensure translation never occurs.Tractable
A successful technique needs to be implementable. New techniques often involve a proof-of-principle prototype that is not suitable for translation. This may involve an off-line reconstruction, a pulse sequence with hard-coded timings/amplitudes, or a post processing algorithm requiring manually pre-processed images.There are multiple conditions for a technique to be tractable: First, others need to understand it. This is needed to implement it efficiently and to relax assumptions in a prototype. This also facilitates broader community acceptance. Second, it needs to be computationally feasible. Additional compute hardware adds cost and may be incompatible with existing architectures. Long computation times are likely unacceptable and efficient real-time implementations may be needed. Third, it needs to be easy to use. The end user does not have your expertise; assume anything obvious to you is not obvious to them.
AI applications may have an advantage over competing techniques in this area. Powerful GPU hardware and optimized compute libraries may allow for tractable compute requirements and pre-trained models reduce the need for complicated manual algorithm coding and optimization. Developers should measure memory and compute times in extreme cases.
Marketable
A technique needs to not only solve an important problem (as described in the “Relevance” section), but one that people are willing to adopt (and typically pay for). The technique needs to have clear indications for use that can be succinctly defined and proven effective with an appropriate study. Ideally, the technique provides a visible advantage over alternative options.The technique also needs a mechanism to reach the end user. The nature of the technique dictates the available options. In some cases – a processing or analysis tool, for example – could involve a stand-alone company selling the solution directly. Often in MRI, partnering with an existing vendor is helpful or even required. In some cases – again consider an analysis tool – partnering with an existing vendor provides access an existing user base, an integrated user experience, and a distribution/sales mechanism. Some techniques, specifically pulse sequences or image reconstruction, almost certainly require partnering with vendors.
Regulatory clearance
Healthcare is a regulated industry and medical devices are subject to regulatory clearance to ensure efficacy and safety before they can be advertised or sold. Generally, regulatory approval requires the previously discussed elements to be in place and documented.AI remains an emerging technology and regulatory requirements are evolving but generally aim to ensure that good design practices were followed so that the device performs as claimed and does not malfunction. The regulatory intent for AI applications is the same as for non-AI applications but proving safety and efficacy can be more challenging than with conventional algorithms.