How to Measure Molecules in Brain Tumors & Why
Brent D Weinberg1 and Hyunsuk Shim2
1Emory University, United States, 2Radiation Oncology, Emory University, Atlanta, GA, United States
1Emory University, United States, 2Radiation Oncology, Emory University, Atlanta, GA, United States
Synopsis
Brain tumors are a challenging diagnosis that depend heavily on imaging, but routine MRI sequences including diffusion (DWI), FLAIR, and post-contrast T1 have their limitations. MRS has the capability to measure molecular concentrations non-invasively and without a contrast injection. Whole brain spectroscopic MRI (sMRI) has the potential to improve the clinical impact of MR spectroscopy on diagnosis and treatment of brain tumors by guiding surgical and radiation management in a way that can improve patient outcomes. Future developments may bring further applications of this technology beyond brain tumors and into other categories of disease.
Introduction
Brain tumors are a challenging diagnosis that depend heavily on imaging, including at the time of initial diagnosis, treatment planning, and longitudinal patient follow-up. At each step of the way, imaging, and in particular, MRI is critical and is widely used. Routine MRI sequences including diffusion (DWI), FLAIR, and post-contrast T1 are key for the evaluation and follow-up of brain tumors. However, these approaches have their limitations in that making the diagnosis can be challenging both at the time of initial diagnosis and follow-up. Advanced imaging techniques including perfusion weighted imaging (PWI), PET, and MR spectroscopy (MRS) have the potential to improve the specificity of diagnoses and improve treatment selection and follow-up. Of these techniques, MRS has the capability to measure molecular concentrations in tissue non-invasively and without a contrast injection. While technical limitations have prevented spectroscopy from reaching the mainstream of clinical care for brain tumors, recent advances in speed, brain coverage, and resolution of 3D whole brain spectroscopic MRI (sMRI) has the potential to be much more impactful in brain tumor care.Current state of the art
In clinical practice, the use of MRS is limited to clinically available sequences provided by scanner vendors. One of two techniques, single voxel spectroscopy or multi-voxel 2D spectroscopy, is typically used and limited to a voxel size of approximately 1 cm3. This can be used as requested by radiologists physicians caring for brain tumors patients. While MRS has potential in helping differentiate disease processes with overlapping imaging appearance (such as primary brain tumors, demyelinating disease, and lymphoma), overlap between spectroscopic findings of these diseases limit its usefulness. Furthermore, they can be cumbersome to apply, requiring a radiologist or technologist to specify voxel positioning. Limitations in the time required and low resolution prevent use of commercially available sequences to evaluate the extent of disease. Current implementation of MRS has clinical impact on a very low number of cases in most practices.Advances in 3D whole brain spectroscopy
Advances in echo planar spectroscopic imaging (EPSI) sequences have been attempted to address many of the current limitations of spectroscopy in the brain. Use of parallel imaging, water and lipid suppression techniques, and specialized coils has improved the ability to obtain high resolution spectroscopic imaging of the brain in reasonable imaging times. This results in 2-4 mm voxels covering 60-80% of the brain volume, making evaluation of regional differences in metabolite concentrations possible. In these instances, a brain can have > 10,000 spectra, bringing up new issues in data processing, quality assessment, and result visualization. We have developed new tools to address these concerns, including a web-based collaborative tool to visualize spectroscopy results and machine learning based tools to remove artifacts and perform peak fitting of key clinical peaks (choline, creatine, and n-acetylaspartate). The result are easy to use maps of metabolite concentrations within and around a tumor bed.Clinical applications in tumor patients
High resolution metabolite maps can be used in brain tumor patients to plan treatment, both for surgical intervention and radiation therapy. Our initial work demonstrated that the choline (Cho) to n-acetylaspartate (NAA) ratio (Cho/NAA) predicted tumor recurrence and had a high correlation with tumor cell density. This inspired the use of sMRI to guide radiation therapy in a dose escalation trial for glioblastoma (GBM) patients. In a small 30 patient, 3 site trial, use of sMRI to treat GBM patients to 75 Gy led to improvements in median survival to 24 months (compared to 16 months in historical controls). We are currently developing a multisite trial through ECOG/ACRIN to test this in a larger number of patients. Additional applications include use of sMRI to select potential biopsy and treatment targets in low grade tumor patients, where there may be little or no enhancement. We are currently performing a pilot trial testing sMRI to guide proton therapy in pediatric high grade gliomas. These studies show the potential for sMRI to guide therapy planning to improve patient outcomes.Ongoing improvements
In light of these initial successes, we continue to improve sMRI through both hardware design and software development to improve sequence speed, resolution, lipid suppression, and motion correction. New hardware design will build shim coils into the head coil to allow improved shimming and brain coverage. Software improvements will further improve processing speed, peak fitting, and artifact reduction. We are also pursuing applications of sMRI beyond brain tumors. Use of a longer TE is allowing for detection of minor metabolites, such as myo-inositol and glutamate, which has potential opportunities for applications in depression, epilepsy, and neurodegenerative disorders.Conclusion
Whole brain spectroscopic MRI (sMRI) has the potential to improve the clinical impact of MR spectroscopy on diagnosis and treatment of brain tumors by guiding surgical and radiation management in a way that can improve patient outcomes. Future developments may bring further applications of this technology beyond brain tumors and into other categories of disease.Acknowledgements
We would like to acknowledge the other members of our team including Hui-Kuo Shu, Eric Mellon, Lawrence Kleinberg, Andrew Maudsley, Mohammed Goryawala, and Hui Han. We received funding from the NIH to support these efforts.References
- Gurbani S, Weinberg B, Cooper L, Mellon E, Schreibmann E, Sheriff S, Maudsley A, Goryawala M, Shu HK, and Shim H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019; 5 (1): 184-191.
- Ramesh K, Gurbani SS, Mellon EA, Huang V, Goryawala M, Barker PB, Kleinberg L, Shu HG, Shim H, and Weinberg BD. The Longitudinal Imaging Tracker (BrICS-LIT):A Cloud Platform for Monitoring Treatment Response in Glioblastoma Patients. Tomography 2020; 6 (2): 93-100.
- Weinberg BD, Kuruva M, Shim H, and Mullins ME. Clinical Applications of Magnetic Resonance Spectroscopy in Brain Tumors: From Diagnosis to Treatment. Radiol Clin North Am 2021; 59 (3): 349-362.
- Zhong J, Huang V, Gurbani SS, Ramesh K, Scott Cordova J, Schreibmann E, Shu HG, Olson J, Han H, Giuffrida A, Shim H, and Weinberg BD. 3D whole-brain metabolite imaging to improve characterization of low-to-intermediate grade gliomas. J Neurooncol 2021.
Figures
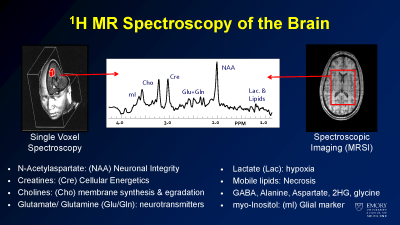
Sample metabolites of interest in brain spectroscopy
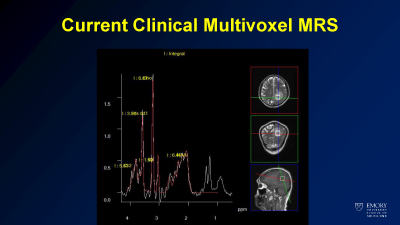
Example of current state of the art clinical spectroscopy
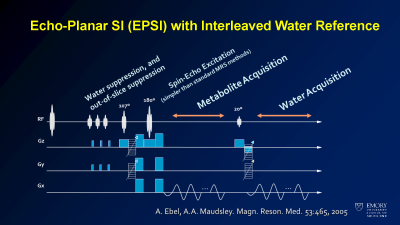
Schematic of EPSI sequence
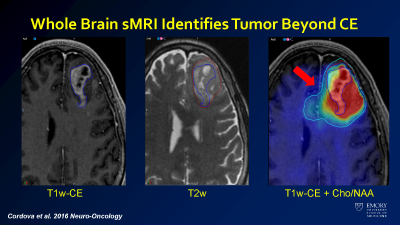
sMRI overlay of choline/NAA shows infiltrating tumor beyond the margins of the tumor as seen on T1 postcontrast and T2 imaging.
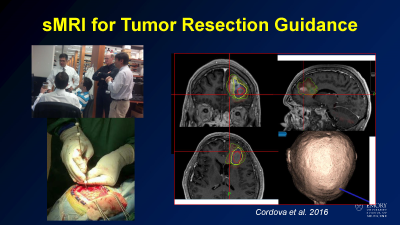
Example case of using sMRI to guid stereotactic brain biopsy
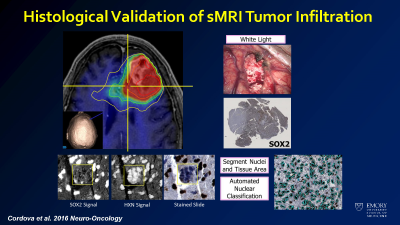
Correlation of sMRI data to tumor density measurements on histology.
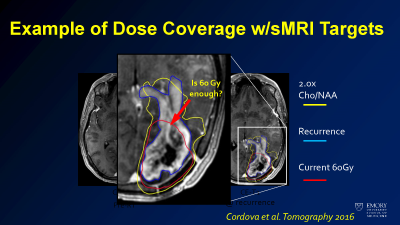
Sample of how sMRI may affect dose targeting overlaid on tumor imaging seen at the time of recurrence.
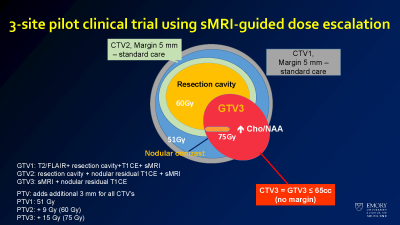
Treatment plan for patients in the sMRI guided radiation therapy for glioblastoma trial.
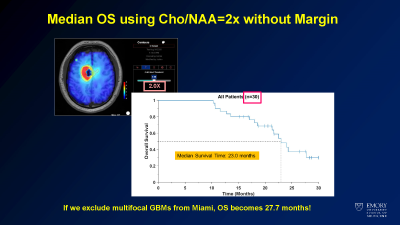
Survival curve from dose escalation trial.
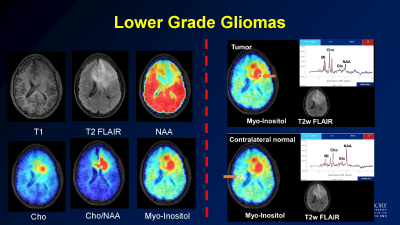
Sample of application of sMRI to determine the extent of low grade glioma.