Artifacts at Low Field
Andrew Webb1
1Leiden University Medical Center, Netherlands
1Leiden University Medical Center, Netherlands
Synopsis
This talk will discuss artifacts and mitigation strategies specifically for low-field and point-of-care MRI systems. Whereas many image artifacts such as fat/water shifts and those arising from medical implants and air/tissue boundaries are predictably much smaller or negligible at low-field, different types of artifacts can potentially occur due to time-dependent magnetic field drift, concomitant gradients, partial volume effects and flow. Gradient strengths are much less than conventional clinical systems, and with magnet inhomogeneities on the orders of hundreds of ppm this means that joint corrections for B0 and gradient non-linearity, together with magnet drift, are required.
Introduction
One disadvantage of MRI as a clinical imaging modality is the expense of system, both in terms of purchase cost and continued maintenance. The magnet must be housed in an RF shielded room, and its operation requires highly-trained technicians. These factors limit its use to late in the healthcare cycle, and its siting to major hospitals. As a result it plays little role in screening in the developed world, and it is estimated that worldwide ~70% of the population has zero access to an MRI scan. Within the past five years there has been a strong push, academically and commercially, to address this issue by developing low-field (<100 mT) MRI systems that have been designed for use in the intensive care unit (ICU), for pediatric imaging in developing countries and as point-of-care devices. Each of these systems is based upon permanent magnet technology, using variously a yoked C-shape magnet, a homogeneous Halbach configuration, or a gradient Halbach configuration. The advantages of low-field MRI include reduced patient contraindications and low SAR, which allows efficient data acquisition using, for example, long echo trains of fully-refocused turbo (fast) spin echoes (TSE). Magnetic susceptibility induced image artifacts are also much reduced compared to clinical field strengths, as are chemical shift artifacts and motion. However, the much lower gradient strengths, higher B0 inhomogeneity, higher B0 temporal drift, and increased electromagnetic interference (EMI) due to the lack of RF shielding all potentially result in greater image artifacts than at conventional field strengths. This talk describes some of these artifacts, and methods for minimizing them.Artifacts: sources and minimization
Magnetic susceptibility: image distortions from medical implants, as well as the associated forces and torques, are much less at low field, as shown for four different structures in Figure 1. Chemical shift: the ~3 ppm difference in chemical shift between fat and water corresponds to an ~6-10 Hz difference at 50-80 mT fields, a value which is much less than the linewidth in the relatively inhomogeneous fields. Therefore, there are no chemical shift artifacts at these field strengths. Gradient non-linearity and B0 inhomogeneity: conventional image distortion correction algorithms require accurate B0 maps which are not possible to acquire directly when the B0 inhomogeneities also produce significant image distortions. One method which has proved to be quite successful is joint estimation, using shifted and non-shifted TSE sequences. For each iteration, ∆B0 maps were reconstructed from the phase difference using Tikhonov regularization, while images were reconstructed using either conjugate phase reconstruction (CPR) or model-based (MB) image reconstruction, taking the reconstructed field map into account. MB reconstructions were furthermore combined with compressed sensing (CS) to show the flexibility of this approach towards undersampling. Results are shown in Figures 2 and 3 in phantoms and in vivo, respectively. Electromagnetic interference: one of the major challenges in POC MRI is that the systems should be designed to operate outside the usual RF shielded environment (Faraday shielded room). Electromagnetic interference (EMI) created either by equipment close to the scanners or consisting of general environmental EM noise can cause artifacts which render images non-diagnostic. For portable MRI devices, some degree of shielding can be built directly around the scanner. However, at least one side of the scanner cannot be shielded to allow patient access. In addition to this opening, the human body acts as an antenna for any external EMI [12] and “guides” this EMI into the imaging region. A conductive mesh cloth can be draped over the patient to reduce the body antenna effect [13], but this set-up can be somewhat uncomfortable and relies on very good contact with any local shielding structures. Many different schemes for EMI reduction have been designed, including most recently one based on external sensors, external sensors plus deep learning or MR-silent modes of a receive coil.Acknowledgements
This work was funded by a Horizon 2020 ERC Advanced Grant (670629).References
1. Cooley CZ, Stockmann JP, Armstrong BD, Sarracanie M, Lev MH, Rosen MS, Wald LL (2015) Two-dimensional imaging in a lightweight portable MRI scanner without gradient coils. Magn Reson Med 73 (2):872-883. 2. Cooley CZ, Haskell MW, Cauley SF, Sappo C, Lapierre CD, Ha CG, Stockmann JP, Wald LL (2018) Design of Sparse Halbach Magnet Arrays for Portable MRI Using a Genetic Algorithm. Ieee T Magn 54 (1). 3. McDaniel PC, Cooley CZ, Stockmann JP, Wald LL (2019) The MR Cap: A single-sided MRI system designed for potential point-of-care limited field-of-view brain imaging. Magnetic Resonance in Medicine 82 (5):1946-1960. 4. Cooley CZ, McDaniel P, Stockmann J, Srinivas SA, Cauley SF, Sliwiak M, Sappo C, Vaughn CF, Guerin B, Rosen MS, Lev MH, Wald LL (2020) A portable brain MRI scanner for underserved settings and point-of-care imaging. arXiv:200413183 [eessIV]. 5. O'Reilly T, Teeuwisse WM, Webb AG (2019) Three-dimensional MRI in a homogenous 27 cm diameter bore Halbach array magnet. J Magn Reson 307. 6. de Vos B, Parsa J, Abdulrazaq Z, Teeuwisse WM, Van Speybroeck CDE, de Gans DH, Remis RF, O'Reilly T, Webb AG (2021) Design, Characterisation and Performance of an Improved Portable and Sustainable Low-Field MRI System. Front Phys-Lausanne 9. 7. Liu YL, Leong ATL, Zhao YJ, Xiao LF, Mak HKF, Tsang ACO, Lau GKK, Leung GKK, Wu EX (2021) A low-cost and shielding-free ultra-low-field brain MRI scanner. Nat Commun 12 (1). 8. Prabhat AM, Crawford AL, Mazurek MH, Yuen MM, Chavva IR, Ward A, Jr WVHV, Timario N, Qualls SR, Helland J, Wira C, Sze G, Rosen MS, Kimberly WT, Sheth KN (2021) Methodology for Low-Field, Portable Magnetic Resonance Neuroimaging at the Bedside. Front Neurol 12. 9. Sheth KN, Mazurek MH, Yuen MM, Cahn BA, Shah JT, Ward A, Kim JA, Gilmore EJ, Falcone GJ, Petersen N, Gobeske KT, Kaddouh F, Hwang DY, Schindler J, Sansing L, Matouk C, Rothberg J, Sze G, Siner J, Rosen MS, Spudich S, Kimberly WT (2021) Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. Jama Neurol 78 (1):41-47. 10. Mazurek MH, Yuen MM, Cahn BA, Rosen MS, Gobeske KT, Gilmore EJ, Hwang D, Kaddouh F, Kim JA, Falcone G, Petersen N, Siner J, Spudich S, Sze G, Kimberly WT, Sheth KN (2021) Low-Field, Portable Magnetic Resonance Imaging at the Bedside to Assess Brain Injury in Patients with Severe COVID-19. Neurology 96 (15). 11. Mazurek MH, Cahn BA, Yuen MM, Prabhat AM, Chavva IR, Shah JT, Crawford AL, Welch EB, Rothberg J, Sacolick L, Poole M, Wira C, Matouk CC, Ward A, Timario N, Leasure A, Beekman R, Peng TJ, Witsch J, Antonios JP, Falcone GJ, Gobeske KT, Petersen N, Schindler J, Sansing L, Gilmore EJ, Hwang DY, Kim JA, Malhotra A, Sze G, Rosen MS, Kimberly WT, Sheth KN (2021) Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage. Nat Commun 12 (1):5119. 12. Li JZ, Nie ZD, Liu YH, Wang L, Hao Y (2017) Evaluation of Propagation Characteristics Using the Human Body as an Antenna. Sensors-Basel 17 (12). 13. O'Reilly T, Teeuwisse WM, de Gans D, Koolstra K, Webb AG (2020) In vivo 3D brain and extremity MRI at 50 mT using a permanent magnet Halbach array. Magn Reson Med doi:10.1002/mrm.28396. 14. Srinivas SA, Cauley SF, Stockmann JP, Sappo CR, Vaughn CE, Wald LL, Grissom WA, Cooley CZ (2022) External Dynamic InTerference Estimation and Removal (EDITER) for low field MRI. Magnetic Resonance in Medicine 87 (2):614-628. 15. Walsh DO (2008) Multi-channel surface NMR instrumentation and software for 1D/2D groundwater investigations. J Appl Geophys 66 (3-4):140-150. 16. Muller-Petke M (2020) Non-remote reference noise cancellation - using reference data in the presence of surface-NMR signals. J Appl Geophys 177. 17. Trushkin DV, Shushakov OA, Legchenko AV (1994) The Potential of a Noise-Reducing Antenna for Surface Nmr Groundwater Surveys in the Earths Magnetic-Field. Geophys Prospect 42 (8):855-862. 18. Larsen JJ, Dalgaard E, Auken E (2014) Noise cancelling of MRS signals combining model-based removal of powerline harmonics and multichannel Wiener filtering. Geophys J Int 196 (2):828-836. 19. Dalgaard E, Auken E, Larsen JJ (2012) Adaptive noise cancelling of multichannel magnetic resonance sounding signals. Geophys J Int 191 (1):88-100. 20. Dalgaard E, Christiansen P, Larsen JJ, Auken E (2014) A temporal and spatial analysis of anthropogenic noise sources affecting SNMR. J Appl Geophys 110:34-42. 21. Hoult DI, Richards RE (2011) The signal-to-noise ratio of the nuclear magnetic resonance experiment. 1976. J Magn Reson 213 (2):329-343.Figures
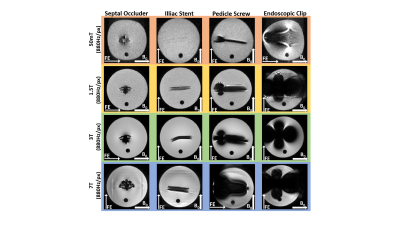
Comparison of image artifacts produced by four different medical implants at four different field strengths, using the same acquisition bandwidth per pixel.
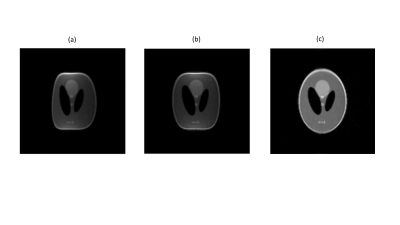
(a) Image of a large Shepp-Logan phantom acquired at 50 mT showing distortions from both B0 and gradient non-linearity. (b) Correction for B0 inhomogeneity. (c) Correction incorporating joint estimation of B0 inhomogeneity and gradient non-linearity.
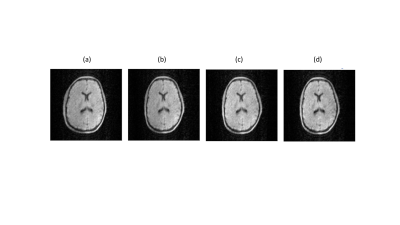
In vivo brain images acquired at 50 mT. (a) No corrections, (b) conjugate phase reconstruction, (c) model based reconstruction and (d) model based compressed sense with a factor-of-two undersampling.