5038
Validation of motion-robust, blood-suppressed, reduced-distortion liver diffusion techniques in a pulsatile motion phantom1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 4GE Healthcare, Boston, MA, United States, 5Electrical and Computer Engineering, University of Wisconsin-Madison, Madison, WI, United States, 6Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Conventional liver diffusion MRI acquisitions suffer from several challenges including pulsatile motion-induced signal voids and B0-induced distortions. In this study, we validated motion-robust, blood-suppressed, reduced-distortion liver diffusion techniques in an anatomically accurate liver phantom including controlled pulsatile motion. By combining optimized motion-compensated, blood-suppressed diffusion waveforms (i.e. MODI) with msEPI acquisitions, superior image quality and quantitative performance can be achieved in the presence of various degrees of compressive tissue motion.
Introduction
Diffusion-weighted (DW)-MRI of the abdomen has various potential applications, including the detection, staging, and treatment monitoring of cancer1. Substantial technical challenges hinder the utility of DW-MRI2–4. Importantly, DW-MRI is sensitive to physiological motion (such as compressive tissue motion induced by cardiovascular pulsation5–8), and susceptibility-induced distortions, particularly when using single shot echo-planar imaging (ssEPI). Recently developed optimized motion-robust DW gradient waveforms enable robustness to pulsatile motion in the liver5–7 and pancreas8 while suppressing blood signal6. In addition, phase-corrected multi-shot EPI (msEPI) techniques9 enable reduced-distortion, high-resolution DW-MRI9,10. Despite early validation of combined M1-optimized-msEPI DW-MRI in volunteers11, highly controlled evaluation as enabled by motion phantoms remains a major validation gap. This work focuses on the validation of the effects of motion-robustness, blood suppression and distortion correction in an anthropomorphic liver model with controlled pulsatile motion.Methods
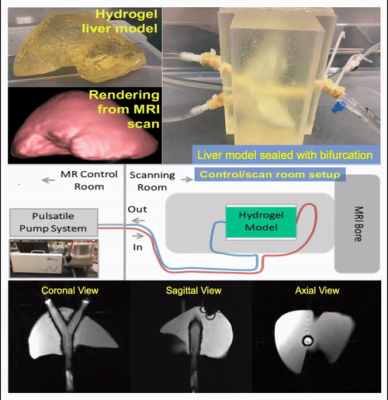
Motion phantom: An anthropomorphic hydrogel liver model surrounds a pulsating tube with bifurcation, while being surrounded by rigid plastic walls (Figure 1). This setup mimics the cyclic motion induced by the cardiovascular-induced compressive tissue motion in the liver. Doped water (3.8L of distilled water with 4g of gadobenate dimeglumine) was pumped through the system, causing deformation of the compliant tube and adjacent hydrogel liver model at a frequency of one hertz. Increased flow amplitude led to increased pulsation of the tube, causing more severe deformation of the liver model.Image acquisition and reconstruction: At various flow velocities (0.1, 0.15, 0.9, 1.1, 1.3 L/min), DW images were acquired at 3T (GE Signa Premier) with high density receive array coils (AIR coil, GE Healthcare), using standard monopolar (MONO, TE = 49 ms) and optimized motion-robust DW gradient waveforms6,7 (TE = 65 ms) with two controlled M1-values (M1-nulled and M1-optimzed with a low M1 of 0.1s/mm). For each type of gradient waveforms, three orthogonal diffusion directions were obtained, each with two b-values (b=50, 500s/mm2, with 2 and 4 repetitions, respectively). For MONO and M1-optimized waveforms, two separate DW-MRI acquisitions using ssEPI and msEPI, respectively, were obtained. Multi-shot EPI was obtained with two shots. Images of msEPI were reconstructed with a multiplexed sensitivity-encoding method9. Non-EPI T2-weighted (T2w) images were acquired with a 2D Fast Spin-Echo sequence as a reference to evaluate geometric distortions in DW-MRI. The same spatial resolution and prescribed locations were used to match the DW-MRI acquisitions.
Analysis: ADC maps were calculated for all DW-MRI series at each flow velocity. Contours of the liver model (excluding the tubing) were drawn from each low-b-value image to extract the liver in the corresponding ADC maps. ADC mean and standard deviation were calculated from areas within 2 cm of the bifurcation in motion-affected slices for each waveform design with ssEPI and msEPI. Distortion level was assessed using the normalized cross-correlation coefficient (CCC)12 between the T2w reference and the low-b-value DW images for each slice.
Results
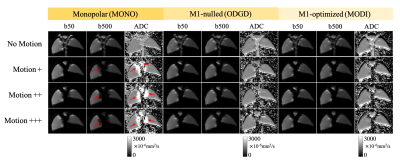
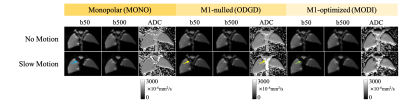
Figure 2 shows the robustness of optimized motion-compensated waveforms to compressive motion. In contrast, severe motion-induced signal dropouts (indicated by red arrows) and ADC bias appear in MONO. Both M1-nulled (ODGD)7 and M1-optimized (MODI)6 methods recover the signal dropouts and produce consistent ADC measurements throughout the liver model.Figure 3 illustrates the suppression of perfusion (blood-mimicking) signals in various DW techniques. With slow flow motion (~0.15L/min), residual bright signal from moving fluid remains in low-b-value images acquired with M1-nulled waveforms, resulting in biased ADC values in the tubing area (yellow arrows). The perfusion signal is suppressed in low-b-value images acquired with M1-optimized waveforms (green arrows) and MONO (blue arrows).
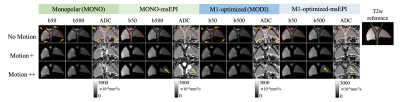
Figure 4 shows the synergy between M1-optimized gradient waveform designs and msEPI with increasing degrees of compressive tissue motion. Single-shot EPI images suffer from severe image distortions (orange arrows). Multi-shot EPI reduces this image distortion. However, msEPI without motion-robust gradients shows artifacts in high-b-value images and biased ADC values (yellow arrows), likely due to motion-induced phase inconsistency across shots. Motion-robust waveforms provide improved phase consistency across shots, and thus M1-optimized-msEPI generates consistent ADC measurements while maintaining low image distortion. CCC comparison shows that the alignment between DW images from MONO-msEPI and M1-optmized-msEPI and the T2w reference is significantly higher than MONO-ssEPI (p=0.01) and M1-nulled-ssEPI (p=0.006), although the alignment between DW images from M1-optmized-msEPI and the T2w reference is not significantly higher than M1-optmized-ssEPI (p=0.052).
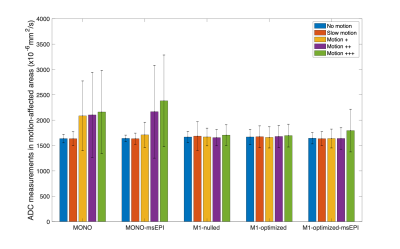
Figure 5 includes the mean and standard deviation of ADC measurements from areas within 2 cm of the bifurcation in motion-affected slices of the liver model. For monopolar waveforms with and without msEPI, ADC mean and standard deviation substantially increase with increasing motion. For the motion-robust methods, ADC mean and standard deviation remain stable with increasing motion, although residual artifacts caused by severe motion (+++) appear in M1-optimized-msEPI.
Discussion
In this study, we validated motion-robust, blood-suppressed, reduced-distortion liver diffusion techniques in a pulsatile motion phantom. By combining optimized motion-compensated, blood-suppressed diffusion waveforms (i.e. MODI) and msEPI acquisition, superior DW-MRI image quality can be achieved in the presence of various degrees of compressive tissue motion, in accordance with previous studies in healthy volunteers11. The proposed phantom validation approach with highly controlled compressive motion may prove valuable in the development and validation of motion-robust MR techniques, in combination with in vivo validation.Acknowledgements
The authors acknowledge support from the NIH (R01 EB030497), from the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation, as well as from the UW Departments of Radiology and Medical Physics. Also, GE Healthcare provides research support to the University of Wisconsin-Madison.References
1. Hernando D, Zhang Y, Pirasteh A. Quantitative diffusion MRI of the abdomen and pelvis. Med Phys. Published online September 23, 2021. doi:10.1002/mp.15246
2. Murphy P, Wolfson T, Gamst A, Sirlin C, Bydder M. Error model for reduction of cardiac and respiratory motion effects in quantitative liver DW-MRI. Magn Reson Med. 2013;70(5):1460-1469. doi:10.1002/mrm.24563
3. Kwee TC, Takahara T, Niwa T, et al. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. Magma N Y N. 2009;22(5):319-325. doi:10.1007/s10334-009-0183-1
4. Naganawa S, Kawai H, Fukatsu H, et al. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 2005;4(4):175-186. doi:10.2463/mrms.4.175
5. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn Reson Med. 2017;77(2):717-729. doi:10.1002/mrm.26166
6. Zhang Y, Peña-Nogales Ó, Holmes JH, Hernando D. Motion-robust and blood-suppressed M1-optimized diffusion MR imaging of the liver. Magn Reson Med. 2019;82(1):302-311. doi:10.1002/mrm.27735
7. Peña-Nogales Ó, Zhang Y, Wang X, et al. Optimized Diffusion-Weighting Gradient Waveform Design (ODGD) formulation for motion compensation and concomitant gradient nulling. Magn Reson Med. 2019;81(2):989-1003. doi:10.1002/mrm.27462
8. Geng R, Zhang Y, Starekova J, et al. Characterization and correction of cardiovascular motion artifacts in diffusion-weighted imaging of the pancreas. Magn Reson Med. 2021;86(4):1956-1969. doi:10.1002/mrm.28846
9. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage. 2013;72:41-47. doi:10.1016/j.neuroimage.2013.01.038
10. Zhang Y, Holmes J, Rabanillo I, Guidon A, Wells S, Hernando D. Quantitative diffusion MRI using reduced field-of-view and multi-shot acquisition techniques: Validation in phantoms and prostate imaging. Magn Reson Imaging. 2018;51:173-181. doi:10.1016/j.mri.2018.04.006
11. Zhang Y, Muehler MR, Geng R, Guidon A, Hernando D. Motion-Robust, Reduced-Distortion Diffusion MRI of the Liver with Optimized Motion Compensated Waveforms and Multi-shot EPI. Int Soc Magn Reson Med Annu Meet 2020 Virtual Conf.
12. Hancu I, Lee SK, Hulsey K, et al. Distortion correction in diffusion-weighted imaging of the breast: Performance assessment of prospective, retrospective, and combined (prospective + retrospective) approaches. Magn Reson Med. 2017;78(1):247-253. doi:10.1002/mrm.26328
Figures