5036
Evaluation of High Resolution Diffusion MRI on the Next-Generation 7T scanner1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Radiology, San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States, 3Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States, 4Advanced MRI Technologies, Sebastopol, CA, United States, 5Siemens Medical Solutions USA, Inc., Melvern, PA, United States, 6Siemens Healthcare, Erlangen, Germany
Synopsis
The newly developed head gradient (200 mT/m Gmax, 900 T/m/s SR) on the new NexGen 7T system provides many potential benefits for high-resolution diffusion imaging. Faster, stronger gradients can significantly reduce diffusion encoding times and TE for up to a 3-fold improvement SNR while also reducing TR and total scan time. We demonstrate these benefits in high resolution diffusion imaging applications with b-values of up to 10,000 s/mm2 at 7T.
Purpose
Both spatial and angular resolution in diffusion weighted EPI are limited by the strength and switching speed of the magnetic gradients used [1]. Conventional body gradients are primarily designed and built for clinical use and limited by peripheral nerve stimulation (PNS). The Next Generation (NexGen) 7T MRI scanner [2] overcomes these limitations and is specifically designed for brain imaging with its novel head gradient coil, novel 128 channel receiver (Rx) and 16 channel transmitter (pTx). Here diffusion weighted SE-EPI pulse sequences are optimized and evaluated to achieve higher spatial and angular resolutions than possible on a conventional 7T scanner.Methods
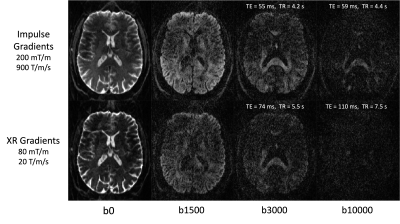
The gradients and other hardware systems on the NexGen 7T scanner (prototype MAGNETOM Terra - Impulse Edition, Siemens Healthcare) were designed and built for the NIH BRAIN Initiative. The PNS-optimized asymmetric head gradient coil, “Impulse” (Siemens Healthcare), achieves Gmax of 200 mT/m and max slew rate (SR) of 900 T/m/s [2,3]. Short term eddy currents (~100 μs) were measured and incorporated into the system’s eddy current compensation gradient pre-emphasis. Subjects were scanned using a custom built 16-channel pTx, 96-channel Rx array [4]. The CMRR C2P Diffusion EPI sequence with a FLEET reference scan was used for improved robustness to motion [5]. In total, five diffusion scans (6-10 mins each) were acquired from a single subject using the following parameters: 53 slices, 120 mm FOV, GRAPPA 3, PF 6/8, 73 diffusion directions (including 8 b0s, 32 b=bmax/2, and 33 b=bmax). Two of the scans were run at maximum gradient performance while another two were run with parameters relaxed to match XR gradient (i.e. Siemens Terra) performance (with either bmax = 3,000 or 10,000 s/mm2 at 1.25 mm isotropic resolution. See Figure 1 for TEs and TRs). The fifth diffusion scan was acquired at 0.8 mm isotropic with a bmax of 1000 s/mm , TE=70 ms and TR=6000 ms. At the time of writing, the C2P implementation of SMS acceleration and GRE FLASH reference scans were not fully compatible on the NextGen software baseline and so were not used. Data were processed using FSL 5.0.11 and the eddy, dtifit, and bedpostx tools [6].Results and Discussion
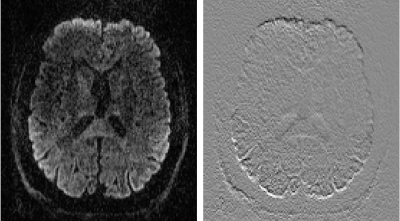
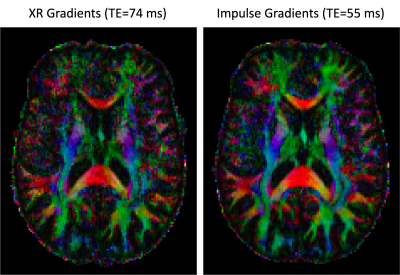
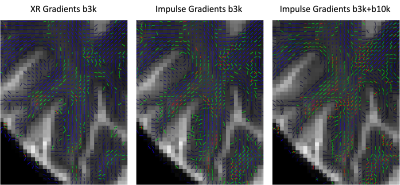
Figure 1 demonstrates the SNR advantage of the Impulse gradients for diffusion weighted imaging at various b-values (top row) relative to images acquired using parameters matched to XR gradient performance (bottom row). Note that the images shown are unaveraged, single TR images. These SNR improvements are due to the shorter TEs afforded by the Impulse gradients and are consistent with the up to 3-fold SNR gain calculated as: exp(TE1/T2)/exp(TE2/T2), using a white matter T2 of 46 ms [7]. Accounting for both short and long term eddy currents in the Siemens’ eddy current correction gradient preemphasis resulted in minimal eddy current distortions in the diffusion weighted images. The residual eddy current distortions were at most only approximately one voxel length towards the most anterior and posterior portions of the brain and were readily corrected by FSL’s eddy tool (Figure 2). The higher SNR afforded by the Impulse gradients also improved estimation of DTI color fractional anisotropy (FA) maps (Figure 3).Figure 4 demonstrates the improved crossing fiber detection in complex white matter regions afforded by the new Impulse gradients. Due to the lower SNR of the data acquired with the XR gradient parameters, very few tertiary fibers are detected (Figure 4 left; red vectors) relative to the data acquired with the Impulse gradients (Figure 4; middle). Enabled by the faster, stronger gradients, our study (to our knowledge) acquired the first bmax=10000 dataset at 7T. Combining this with the bmax=3000 dataset resulted in the best crossing fiber detection performance (Figure 4, right; 2.5-fold more tertiary fibers than bmax=3000 data alone and 4.5-fold more than the XR gradient performance bmax=3000 data).
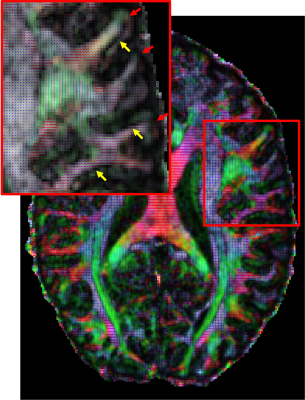
Finally, by utilizing the fast slew rate of the Impulse gradients we were also able to push the spatial resolution to 0.8 mm which is more than a factor of two finer in volumetric resolution compared to prior Human Connectome Project 7T diffusion data [5]. Figure 5 shows the primary diffusion direction map overlayed onto FA for this 0.8 mm data which reveals dark bands of FA not typically seen at lower resolutions [5]. The dark bands tend to be strongest along sulcal banks (yellow arrows; where white matter tracts turn sharply into cortex) and weakest in the portions of gyral crowns where the white matter tracts continue straight into the gray matter (red arrows).
Summary and Conclusion
We have demonstrated multiple benefits of the NexGen 7T for diffusion imaging in terms of reduction in diffusion encoding time, acquisition time, and up to 3-fold improvement in SNR. These increases in SNR, due to the novel “Impulse” gradients, allow for substantially higher spatial and angular resolution (up to b=10,000 s/mm2) and 4.5-fold improved crossing fiber detection performance. With SMS acceleration enabled, we expect to see additional advantages from high channel count head coils in development for the NexGen system, including a 128-channel receive RF coil [8].Acknowledgements
Research supported by the NIH BRAIN Initiative (R01MH111444, U01-EB025162), 1R44MH129278.References
[1] Feinberg et al “Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI” Neuroimage 2018
[2] Feinberg et al “Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays.” ISMRM 2021
[3] Davids et al “PNS optimization of a high-performance asymmetric gradient coil for head imaging” ISMRM 2021
[4] Gunamony et al “A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner” ISMRM 2021
[5] Vu et al “High resolution whole brain diffusion imaging at 7 T for the Human Connectome Project” Neuroimage 2015
[6] Jenkinson et al “FSL” Neuroimage 2012
[7] Yacoub et al “Spin-Echo fMRI in Humans Using High Spatial Resolutions and High Magnetic Fields” Magn Reson Med 2003
[8] Gruber et al “A 128-Channel head coil array for Cortical Imaging at 7 Tesla” ISMRM 2021
Figures