5033
Combined Stiffness and PDFF Phantom for Quality Assurance of Quantitative Magnetic Resonance Elastography and Fat Fraction Imaging1Calimetrix, Madison, WI, United States, 2Mechanical Engineering, University of Wisconsin, Madison, WI, United States, 3Radiology, University of Wisconsin, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin, Madison, WI, United States, 5Medical Physics, University of Wisconsin, Madison, WI, United States, 6Medicine, University of Wisconsin, Madison, WI, United States, 7Emergency Medicine, University of Wisconsin, Madison, WI, United States
Synopsis
MRI proton density fat fraction (PDFF) and MR elastography (MRE) methods are frequently used to evaluate patients with known or suspected NAFLD or NASH. Reference standards (phantoms) are needed for quality assurance of these methods. Currently available phantoms mimic only a single tissue property. A phantom that simultaneously mimics PDFF and tissue stiffness would more realistically mimic NAFLD/NASH tissue and would enable robust and convenient quality assurance. In this work, a chemical formulation and phantom configuration were developed for a multi-layer combined PDFF-MRE phantom, in which each layer simultaneously mimics different PDFF and stiffness values.
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects approximately 100 million Americans, and is characterized by elevated liver fat[1]. A subset of NAFLD patients develop nonalcoholic steatohepatitis (NASH), which if left untreated can lead to end stage liver disease (cirrhosis), liver failure, and hepatocellular carcinoma (HCC)[1-5].Liver biopsy can be used to diagnose and monitor treatment of NAFLD and NASH. However, biopsy carries non-negligible risk and is prone to sampling and observer variability[6,7]. In the past decade, MRI proton-density fat fraction (PDFF) and MR elastography (MRE) tissue stiffness measurements have emerged as alternatives to biopsy for quantitative assessment of liver fat and fibrosis. However, the rapid development and adoption of these quantitative MRI methods at thousands of unique clinical and research sites has created the need for quantitative MRI reference standards (phantoms) that enable robust quality assurance to ensure precise measurement of liver PDFF and stiffness.
This study was aimed at designing a multi-PDFF, multi-stiffness quantitative phantom in a single compact unit, by developing chemical formulations for composite materials that simultaneously span the clinically relevant ranges of liver PDFF and liver tissue stiffness. Such a phantom requires: (1) A base material that can be modified to predictably modulate mechanical stiffness and produces a water-like signal for fat-water imaging, (2) A substance that produces MRI fat signal, and (3) A compact configuration that enables repeatable quantitative stiffness measurements by meeting minimum wavelength requirements based on MRE excitation frequency[8].
Methods
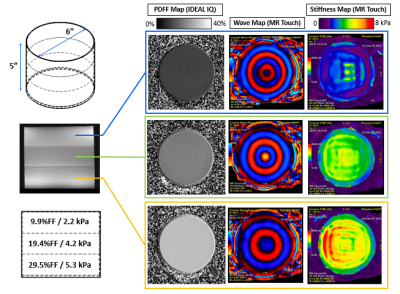
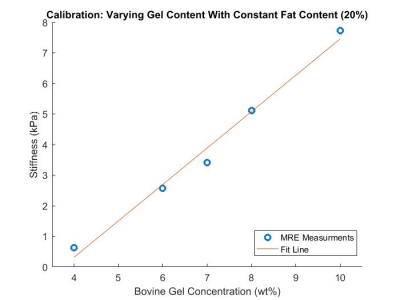
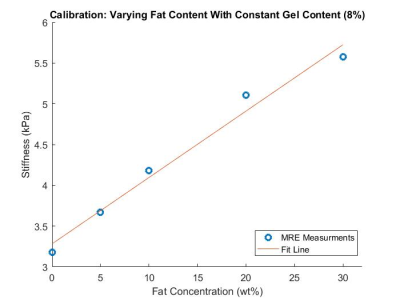
A three-layer multi-PDFF, multi-stiffness quantitative phantom (Figure 1) was developed by stacking layers of emulsified bovine gelatin and oil at various concentrations.First, base materials were calibrated to MRI-PDFF and MRE stiffness metrics. To do this, bovine gelatin powder and surfactant were mixed into water at gel concentrations of 4-12wt%. The solutions were heated to 45oC. Oil was then added at concentrations ranging from 0-30wt% before the solutions were emulsified with a homogenizer. The emulsions were poured into cylindrical high density polyethylene containers with a height of 5 inches and a diameter of 6 inches. In total, 10 calibration phantoms were created with the following nominal gel%/oil% emulsion pairs: 4/20, 6/20, 7/20, 8/20, 10/20, 12/20, 8/0, 8/5, 8/10, 8/20, 8/30.
The calibration phantoms were imaged on a 3.0T clinical MRI system (Signa Premier, GE Healthcare, Waukesha, WI) using a commercial MR elastography system (Resoundant, Rochester, MN USA) and 48-channel head coil. The MRE passive driver and a rubber friction pad were fixed to the flat cylindrical surface of each phantom by wrapping the phantom-driver unit with a neoprene belt. MRE stiffness images were acquired using a spin-echo EPI-based MR elastography acquisition (MR Touch, GE Healthcare) (20cm FOV, 32x32 matrix, 8mm slice thickness, 3 slices, 60Hz frequency, 10% amplitude).
Quantitative PDFF maps were acquired using an investigative version of a confounder-corrected chemical shift encoded MRI acquisition (IDEAL IQ, GE Healthcare) (28cm FOV, 2x2mm resolution, 2mm slice thickness, 66 slices, TR=9.5, FA=4o, first/last echo=1.15/7.76, 8 echoes interleaved in two echo trains).
After calibration data were analyzed, target PDFF (10-30% PDFF) and stiffness (2-6 kPa) ranges were selected for a multi-layer PDFF/stiffness phantom. The stacked phantom was created by making one emulsion, pouring it, letting it cool and cure, and then repeating the process for each subsequent layer. This stacked-layer design was selected so that the in-plane dimension of each PDFF/stiffness layer was the full diameter of the phantom. Maximizing the in-plane dimension is important to ensure enough mechanical wavelengths across the phantom for accurate MRE values to be calculated[8]. The manufacturing process required a total elapsed time of three days (one day per layer) due to the need for each layer to cure completely before pouring the next layer. The multi-layer phantom was imaged with the same IDEAL IQ and MR Touch protocols described above.
Results
A multi-layer MRI/MRE phantom was created with three compartments of unique PDFF/Stiffness formulations measuring 9.9%/2.2kPa, 19.4%/4.2kPa, and 29.5%/5.3kPA, as shown in Figure 1. Calibration relationships were also characterized between combined oil/gelatin quantities and PDFF/Stiffness metrics, as shown in Figures 2 and 3.Discussion
In this work, we successfully created a multi-PDFF, multi-stiffness quantitative phantom in a single compact unit. The PDFF and stiffness values span the clinically relevant range of liver fat and stiffness values seen in patients with NAFLD/NASH in a stacked-compartment configuration.MRI methods to quantify liver fat and tissue fibrosis are frequently used together in studies to evaluate patients with known or suspected NAFLD or NASH. Currently available phantoms mimic only one tissue property. We have developed a phantom with chemical formulations that can be modulated to represent both PDFF and stiffness simultaneously. Furthermore, we developed a stacked-compartment design that allows for repeatable quantitative MRE measurements by satisfying minimum wavelength-to-diameter requirements[8]. This quantitative phantom more realistically mimics the liver tissue of NAFLD/NASH patients and facilitates robust and convenient quality assurance of PDFF and MRE methods. In future work, we plan to further validate the phantom materials with non-image-based mechanical testing, and plan to test and optimize the longitudinal stability of the phantoms.
Conclusion
A combined stiffness and PDFF phantom was developed through optimized chemical formulations and design of a robust configuration to facilitate quantitative MRI and MRE quality assurance.Acknowledgements
The authors wish to acknowledge Phil Rossman for helpful discussions regarding MRE phantom development, and GE Healthcare who provides research support to the University of Wisconsin.References
[1] A. Ahmed, R. J. Wong, and S. A. Harrison, "Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes," (in eng), Clin Gastroenterol Hepatol, vol. 13, no. 12, pp. 2062-70, Nov 2015, doi: 10.1016/j.cgh.2015.07.029.
[2] S. Hoodeshenas, M. Yin, and S. K. Venkatesh, "Magnetic Resonance Elastography of Liver: Current Update," (in eng), Top Magn Reson Imaging, vol. 27, no. 5, pp. 319-333, Oct 2018, doi: 10.1097/RMR.0000000000000177.
[3] M. Lai and N. H. Afdhal, "Liver Fibrosis Determination," (in eng), Gastroenterology clinics of North America, vol. 48, no. 2, pp. 281-289, 2019, doi: 10.1016/j.gtc.2019.02.002.
[4] D. C. Rockey, P. D. Bell, and J. A. Hill, "Fibrosis--a common pathway to organ injury and failure," (in eng), N Engl J Med, vol. 372, no. 12, pp. 1138-49, Mar 19 2015, doi: 10.1056/NEJMra1300575.
[5] S. B. Reeder and C. B. Sirlin, "Quantification of liver fat with magnetic resonance imaging," (in eng), Magn Reson Imaging Clin N Am, vol. 18, no. 3, pp. 337-57, ix, Aug 2010, doi: 10.1016/j.mric.2010.08.013.
[6] V. Ratziu et al., "Sampling variability of liver biopsy in nonalcoholic fatty liver disease," (in eng), Gastroenterology, vol. 128, no. 7, pp. 1898-906, Jun 2005, doi: 10.1053/j.gastro.2005.03.084.
[7] S. Ravindran, S. H. Hancox, and D. C. Howlett, "Liver biopsy: past, present and future," (in eng), British journal of hospital medicine (London, England : 2005), vol. 77, no. 2, pp. 90-95, 2016, doi: 10.12968/hmed.2016.77.2.90.
[8] S. P. Arunachalam et al., "Quantitative 3D magnetic resonance elastography: Comparison with dynamic mechanical analysis," (in eng), Magn Reson Med, vol. 77, no. 3, pp. 1184-1192, 03 2017, doi: 10.1002/mrm.26207.
Figures