5027
Spatial encoding with a 48-ch Transcranial Magnetic Stimulation coil array: Application to diffusion imaging
Jason Peter Stockmann1,2, Lucia Navarro de Lara1, Mohammed Daneshzand1, Qinglei Meng1, Congyu Liao3, Hong-Hsi Lee1,2, Lawrence L Wald1,2, Berkin Bilgic1,2, Susie Huang1,2, and Aapo Nummenmaa1,2
1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Lucas Center, Stanford University, Stanford, CA, United States
1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Lucas Center, Stanford University, Stanford, CA, United States
Synopsis
We show that multi-channel TMS (mTMS) probe arrays can be used to generate B-fields inside the head for spatial encoding in concurrent TMS/MRI studies. We assess the field-synthesis capability of a 48-ch mTMS array for diffusion imaging and spatial encoding more broadly. Proof-of-concept diffusion imaging experiments on a 3T scanner using a 3-axis TMS probe achieve spatially-varying b-values >500 s/mm2 at the depth of the peripheral gray matter using only 4A of current. We consider the potential for scaling up to 200A for studies of gray matter microstructure.
INTRODUCTION
TMS has been employed for studying the response of specific brain circuits using concurrent BOLD-fMRI acquisition [1]. MR-compatible multi-channel transcranial magnetic stimulation (mTMS) arrays have been proposed as a means of dynamically steering E-fields across the cortical surface. These systems employ multi-turn coils with high current-carrying capacity close to the head that are capable of producing brief magnetic field pulses with local field strengths up to several Tesla. Therefore, such mTMS arrays may also provide means for synthesizing nonlinear B-field profiles inside the head for spatial encoding purposes in MRI. Moreover, with their low inductance and minimal coupling to other bore structures (i.e., low eddy currents) TMS element currents can be rapidly switched using comparatively low-voltage amplifiers. mTMS coils have a low duty-cycle for stimulation in TMS-fMRI experiments and are thus available for spatial-encoding during most of the scan duration.A 48-ch mTMS system was recently explored as a way to perform high-spatial order, dynamic B0-shimming of the brain [2]. This approach is analogous to other dual-purpose coil arrays such as integrated RF receive-arrays that have been modified to add DC current-carrying capability for B0 shimming [3,4]. However, the multi-turn TMS coils provide much higher B0 field offsets per ampere of current used, opening up greater possibilities for spatial encoding.
In this work we explore the feasibility of using mTMS arrays for spatial encoding more broadly using simulations as well as proof-of-concept experiments. As an example application, we use a single 3-axis TMS coil to perform diffusion encoding on a phantom. Compared with body gradients, mTMS coil array is unlikely to provide superior diffusion encoding across the whole brain, but in the gray matter, the mTMS has the potential to exceed state-of-the-art gradient coils. Since a limited number of investigators have access to high-performance Connectome-style gradient systems for studying microstructure in vivo humans [5], mTMS could provide an alternative tool for diffusion imaging as a stand-alone ‘insert’ that is relatively easy to install on any clinical scanner (and is removable). Furthermore, in addition to diffusion encoding the mTMS system can be used to ‘boost’ the performance of the linear imaging gradients by offering supplementary spatial encoding capabilities [6].
METHODS
First, we calculated the magnetic field Bz components of the individual 3-axis coils using the BEM-FMM toolkit [7] assuming 1A current. Subsequently, we employed the target-field approach similarly to the multicoil shimming approach to investigate the capability of the 3-axis coil array to synthesize various field patterns across an axial slice assuming maximum current amplitude of 200 A/channel.We then demonstrated proof-of-concept diffusion-encoding in a gel phantom using a prototype MR-compatible 3-axis TMS probe [8] on a Siemens Skyra 3T scanner. To provide high receive-sensitivity and enable parallel-imaging, we used an in-house-built 28-ch RF receive-array that incorporates access holes so TMS probes can rest on the scalp (Figure 1). Currents in each TMS channel are driven by an open-source shim-amplifier capable of driving up to 4 A/channel [9]. B0 field-maps of each TMS element are obtained using a vendor-provided two-echo field mapping sequence. Diffusion imaging is performed using Stejskal-Tanner-style monopolar diffusion lobes with a spin-echo echo-planar imaging pulse sequence (2.3mm in-plane, 4mm slices). The shim amplifiers play diffusion-encoding pulses upon receiving a trigger from the scanner. Using diffusion-weighted images and the known ADC of the phantom, we computed spatially-varying b-value maps.
RESULTS
The simulated results in Figure 2A shows that close to the wire windings the z-element produces a Bz of about 0.5mT/A that rapidly decreases as a function of distance. This natural decay gives rise to a strong local gradient: for the z-coil, the field drops from ~0.4mT to zero over ~4cm distance, resulting in a gradient strength of ~10mT/m per 1A of current. As shown in Figure 2B-C, the 3-axis coil array can also produce linear and nonlinear ‘global’ gradient patterns across an axial slice with somewhat reduced efficiency when compared with the natural gradients of two z-coils that results in a local gradient or ~1T/m when driven with 200 A/channel.Figure 3 shows the experimental setup. Figure 4 shows example B0 field-maps acquired with 1A of current on the X-and-Y-elements of the 3-axis probe. Figure 5 shows a b-value map computed from diffusion-weighted-images acquired with 4A of current on the z-element.
DISCUSSION
Our results show that an array of small-diameter (~5cm) 3-axis TMS coils can produce a wide variety of spatial encoding fields. The key insight is that coils produce very strong ‘natural’ field gradients per ampere of applied current when placed next to the scalp. In addition, when all elements are combined, ‘global’ linear and non-linear encoding gradients can be synthesized for various spatial encoding tasks. A particularly interesting application of the system would be diffusion imaging. While the b-values generated by the mTMS coils are spatially-varying, they potentially surpass existing “Connectom” gradient coils in the gray matter. Preliminary calculations show that they could achieve local gradients of 1T/m or higher, providing a useful tool for studying the microstructure of gray matter. In the full 48-ch mTMS setup, the encoding field could be optimized for an anatomical region of interest.Acknowledgements
The authors gratefully acknowledge support from NIH NIBIB R01MH111829, R00EB021349, and P41EB030006. The authors thank Lincoln Craven-Brightman for help with setting up validation experiments.References
- Ruff, Christian C., Felix Blankenburg, Otto Bjoertomt, Sven Bestmann, Elliot Freeman, John-Dylan Haynes, Geraint Rees, Oliver Josephs, Ralf Deichmann, and Jon Driver. "Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex." Current Biology 16, no. 15 (2006): 1479-1488.
- Stockmann JP, Navarro de Lara L, Wald L, Nummenmaa A. Feasibility of using a 3-axis multi-channel TMS coil array for B0 shimming of the brain at 3T. In: Proc. Int. Soc. Magn. Reson. Med. ; 2020. p. 1132.
- Stockmann JP, Witzel T, Keil B, Polimeni JR, Mareyam A, LaPierre C, Setsompop K, Wald LL. A 32-channel combined RF and B0 shim array for 3T brain imaging. Magn. Reson. Med. 2016;75(1):441-51.
- Truong, Trong-Kha, Dean Darnell, and Allen W. Song. "Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain." NeuroImage 103 (2014): 235-240.
- Huang SY, Witzel T, Keil B, Scholz A, Davids M, Dietz P, Rummert E, Ramb R, Kirsch JE, Yendiki A, Fan Q, Tian Q, Ramos-Llordén G, Lee HH, Nummenmaa A, Bilgic B, Setsompop K, Wang F, Avram AV, Komlosh M, Benjamini D, Magdoom KN, Pathak S, Schneider W, Novikov DS, Fieremans E, Tounekti S, Mekkaoui C, Augustinack J, Berger D, Shapson-Coe A, Lichtman J, Basser PJ, Wald LL, Rosen BR. Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome. Neuroimage. 2021 Nov;243:118530. doi: 10.1016/j.neuroimage.2021.118530. Epub 2021 Aug 28. PMID: 34464739.
- Cho J, Liao C, Tian Q, et al. Highly Accelerated EPI with Wave Encoding and Multi-shot Simultaneous Multi-Slice Imaging. arXiv [eess.IV] [Internet] 2021
- Makarov SN, Wartman WA, Daneshzand M, Fujimoto K, Raij T, Nummenmaa A. A software toolkit for TMS electric-field modeling with boundary element fast multipole method: an efficient MATLAB implementation. J Neural Eng. 2020 Aug 4;17(4):046023. doi: 10.1088/1741-2552/ab85b3. PMID: 32235065.
- Navarro de Lara LI, Golestanirad L, Makarov SN, Stockmann JP, Wald LL, Nummenmaa A. Evaluation of RF interactions between a 3T birdcage transmit coil and transcranial magnetic stimulation coils using a realistically shaped head phantom. Magn. Reson. Med. 2020;84:1061–1075. doi: 10.1002/mrm.28162.
- Arango N, Stockmann J, Witzel T, Wald L, White J. Low-cost, open-source, flexible current feedback-controlled coil driver circuit for matrix B0 shim coils and related applications. In: Proceedings of the ISMRM, 2016, p. 1157.
Figures
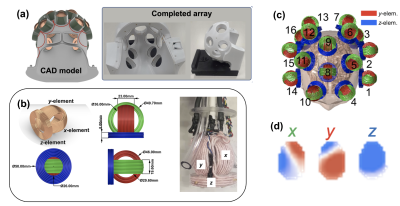
Figure 1. mTMS and associated RF hardware. (a) 28-ch RF receive array with access ports for sliding TMS probes onto the surface of the head. (b) Design of 3-axis TMS probe along with hardware prototype. (c) 48-ch arrangement of TMS coils over the surface of the head. (d) B0 field maps for one 3-axis probe showing the spatially-independent magnetic field profiles of the 3 probe axes.
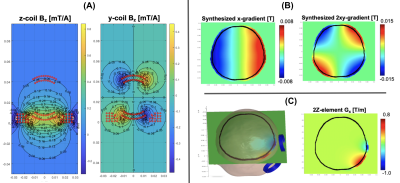
Figure 2 (A): The Bz-components for the z-coil (left panel) and y-coil (right panel) per 1 A applied current.(B): Synthesized linear (left) and non-linear gradient patterns (right) when the currents in the coils were limited to 200 A/channel. (C): The ‘natural’ gradients of two neighboring z-coils (left) when both driven with 200 A/channel with the same polarity (right).
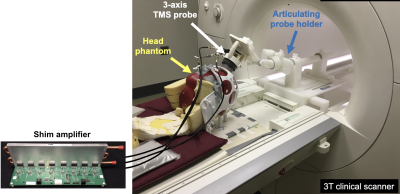
Figure 3. Experimental setup for proof-of-concept diffusion encoding experiments on a 3 Tesla scanner. An in-house 8-ch shim amplifier is used to drive up to 4A in each axis of a prototype mcTMS probe placed on the surface of a gel phantom. Triggers in a spin-echo EPI diffusion imaging sequence are used to play out monopolar diffusion encoding waveforms on the amplifiers. [Note: The 28-ch Rx array is not shown in this photo but was used in subsequent experiments]
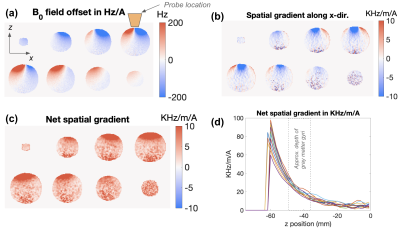
Figure 4. Coronal image slices showing example B0 field generated when the X and Y channels of 3-axis TMS probe are driven with 1A current. (b) and (c) show the x-component of the spatial field gradient as well as the net gradient, respectively. (d) Depth profiles of the net field gradient show sufficient depth of penetration to provide diffusion encoding in the gray matter.
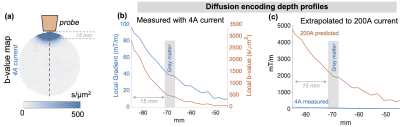
Figure 5: (a) Early experimental results on a phantom with 4A of current driving the z-axis of the prototype 3-axis TMS probe. (b) The local b-value at the depth of gray matter is on the order of ~500 s/mm2. (c) Predicted encoding field with 200A reaches 2000 mT/m within gray matter.
DOI: https://doi.org/10.58530/2022/5027