5024
Noncontact in-bore cardiopulmonary sensing using CW doppler radar1Radiology, Stanford University, Stanford, CA, United States, 2GE healthcare, Cleveland, OH, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Noncontact vital sensing applications have great potential to aid in patient care. We leverage a reconfigurable SDR to assess the feasibility of in-bore cardiopulmonary sensing using CW doppler radar that operates independently of the MRI system and data acquisition.
Introduction
This study investigates the feasibility of noncontact CW doppler radar cardiopulmonary sensing during MRI that operates independently of MRI signal acquisitions. Respiratory bellows, ECG or PPG are widely used during MRI for cardiac and respiratory motion correction, but often add setup time, or cause patient discomfort and interference with other in-bore apparatus. The efficacy of such devices in a harsh electromagnetic environment may be limited 1 or infeasible for certain patient populations 2. In non-contact doppler radar, the group delay caused by tiny physiological motions of breathing and heartbeat creates detectable phase shifts in the reflected signal 3. In this work, we leverage a reconfigurable SDR (bladeRF 2.0, Nuand) to detect in-vivo cardiopulmonary signals within the MR bore during an MRI scan. The radar system adopts the multichannel receiver architecture running at 2.4GHz using the bladeRF and two in-house antennas. The sensed radar vital signs were validated with synchronized system references.Method
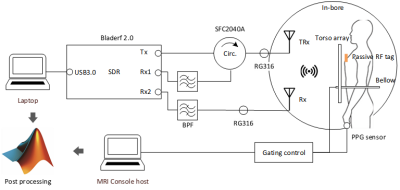
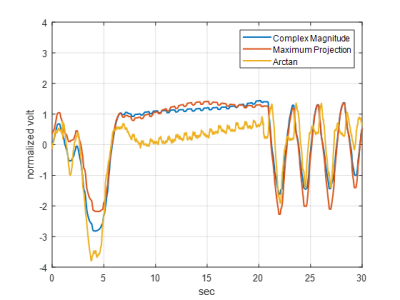
Figures 1 & 2 show the schematic and photograph of the radar system and its integration in a 3T wide bore system (MR750w, GE). Inside the scan room, two antennas were attached to the bore ceiling symmetric with iso-center, and adjacent to each other above the subject’s landmark position. These antennas were remotely connected by RF coax cables to a bladeRF, located in the console room. A healthy volunteer lay in the supine position at the cardiac landmark, with a torso anterior array (Air-coil, GE). A passive RF tag was also attached on the subject chest to enhance backscattered waves. About 20cm of gap existed between the antenna and the subject’s chest wall. A single slice FIESTA MRI sequence (11s) was prescribed, and the system bellows and PPG pulse sensor were wired to the subject. These vital signs sensors were recorded by the MRI gating control. The radar transmitted a 2.4GHz monotone with estimated power of 0.23µW to the antenna terminal. The received signal was first demodulated with a 50kHz Rx offset from transmit LO, and then numerically down-converted to baseband by a digital down converter (ddc) Matlab subscript. Radar data was independently acquired for 30 seconds, which included 11s of sequence run-time. The baseband signals were pre-conditioned to eliminate artifacts made by MR system interference prior to the vital signs processing in figure 3 (b). The preconditioned time series was processed by three different methods (complex magnitude, maximum projection, and arctan) for the reconstruction fidelity check of figure 4. To extract heartrate, the singular spectral analysis (SSA) algorithm 4 was applied over the breath holding time window after high pass filtering at 0.8Hz. The processed time series was validated by the MR-synchronized PPG/bellows signals (figure 5).Results
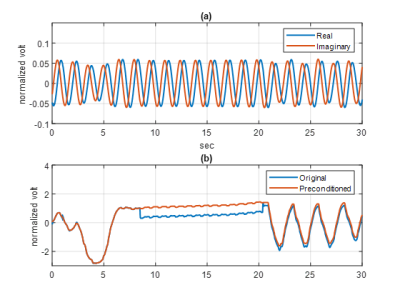
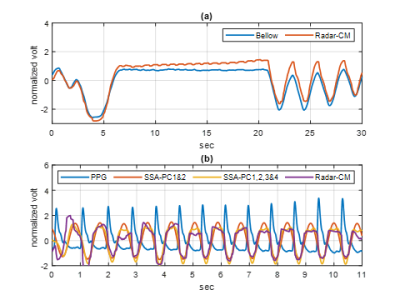
Figure 3 (a) shows the complex demodulated time series real (blue) and imaginary (red) components over 30 seconds. The temporal resolution after decimation was 15.7ms, which was fast enough to capture the cardiac cycles. Each component of the complex data exhibited a residual frequency offset with period of 1.49s, arising from local oscillator frequency synthesizer roundoff. The complex magnitude of the time series before (blue) and after (red) the artifact correction are shown in figure 3 (b). The signal blips from MR system interference were successfully removed (red) for further vital signs processing. Figure 4 provides the artifact-corrected time series by the three reconstruction algorithms. In all cases, the breath hold intervals (plateau) were well delineated and in good agreement. The relatively small peaks within the plateau were also evident and with periodicity consistent with the heart rate of the subject. Figure 5 (a) shows the complex magnitude of the radar signal time-aligned with the system bellows, demonstrating clear delineation of the breath holding window in both. The depicted SSAs denoised the original radar signal and were aligned well with the PPG data over the window in figure 5 (b).Discussion
CW radar is known to consume low power and to have non-stringent bandwidth requirements. In addition, implementation is relatively simple compared to pulsed radar 5. The SDR used in this study provided sufficient signal fidelity even with sub µW transmit power to reconstruct a vital-signs time series during MR cardiac scan operation. The complex magnitude reconstruction yielded a clear cardiopulmonary signature in the presence of residual phase noise of the radar system. Other reconstruction approaches may require precise phase offset control. Furthermore, the capability of null point free reconstruction with the quadrature receiver allows freedom in antenna-subject separation, in support of diverse subject populations. The SSA reconstruction was found to be helpful for heart rate extraction by reducing inherent noise other than the target quasi-periodic signals 6. In system integration, the radar signal encounters some interference by the MR scan operation. During cardiac sequence acquisition, the interference appeared as data blips, which could be addressed by the correction algorithm.Conclusion
In this study, noncontact in-bore vital sign sensing was demonstrated with CW doppler radar in a 3T MRI, and its feasibility was confirmed for in-vivo application with the existing respiratory/pulse sensorsAcknowledgements
We thank GE Healthcare for research support, and received funding from NIH grants R01 EB019241, U01EB029427, R01EB012031, U01EB026412References
1. Nacif MS, Zavodni A, Kawel N, Choi EY, Lima JA, Bluemke DA. Cardiac magnetic resonance imaging and its electrocardiographs (ECG): tips and tricks. Int J Cardiovasc Imaging. 2012;28(6):1465-1475. doi:10.1007/s10554-011-9957-4
2. Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magn Reson Med. 2016 Jul;76(1):197-205. doi: 10.1002/mrm.25858. Epub 2015 Jul 29. PMID: 26220204; PMCID: PMC4732937.
3. C. Li, V. M. Lubecke, O. Boric-Lubecke and J. Lin, "A Review on Recent Advances in Doppler Radar Sensors for Noncontact Healthcare Monitoring," in IEEE Transactions on Microwave Theory and Techniques, vol. 61, no. 5, pp. 2046-2060, May 2013, doi: 10.1109/TMTT.2013.2256924.
4. Deng, Cheng, "Time Series Decomposition Using Singular Spectrum Analysis" (2014). Electronic Theses and Dissertations. Paper 2352. https://dc.etsu.edu/etd/2352
5. Olga Boric-Lubecke; Victor M. Lubecke; Amy D. Droitcour; Byung-Kwon Park; Aditya Singh, "Radar Principles," in Doppler Radar Physiological Sensing , IEEE, 2016, pp.21-38, doi: 10.1002/9781119078418.ch2.
6. J. Zabalza,
J. Ren and S. Marshall, "Singular Spectrum Analysis for effective noise
removal and improved data classification in Hyperspectral Imaging," 2014
6th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote
Sensing (WHISPERS), 2014, pp. 1-4, doi:
10.1109/WHISPERS.2014.8077583.
Figures