5020
Spikes-on-demand. A simple method to generate realistic spikes to test artifact detection algorithms1Cleveland Clinic, Cleveland, OH, United States
Synopsis
Quality assurance (QA) protocols can and should be used to proactively detect low-level spiking and other artifacts early so that these problems can be remedied before becoming more debilitating and adding significant overhead to the image analysis workflow. However, one generally needs corrupted data sets to test the accuracy of the novel algorithm. This prevents researchers from taking a proactive stance of establishing these QA protocols before corruption of data sets occurs. Here, we detail a simple method to generate spikes reliably and reproducibly to generate corrupted data that can be used to test and debug any new QA algorithms.
Introduction
Imaging modalities such as fMRI and ASL are sensitive to small changes in signal. Subtle artifacts can make such imaging inaccurate.However, when testing new algorithms to monitor performance during Quality Assurance (QA), one generally needs corrupted data sets to test the accuracy of the novel algorithm. This prevents researchers from taking a proactive stance of establishing these QA protocols before corruption of data sets occurs. This may require retrospective correction of tens or more of data sets, which is a costly and time intensive endeavor. This is particularly true in the case of spike noise, which can begin as intermittent and almost imperceptible signal modulations before worsening over time.
While a number of automated detection algorithms have been proposed 1,2,3,4,5,6,7,8,9, testing these algorithms can be difficult without a sample of images containing artifact. Injecting spikes into raw data is a standard approach, but can be impossible without access to proprietary image reconstruction code. Here we discuss a simple method that reliably produces spike noise artifacts for this testing spike detection algorithms.
Methods
Imaging was performed on a Siemens 3T Prisma (Siemens Healthineers, Erlangen) with a standard 20 channel Head/Neck array. Simultaneous Multi-Slice (SMS)10,11 echo planar imaging (EPI) acquisitions were acquired (axial, 192 mm x 192 mm FOV, 94 x 94 matrix, 45 slices, 3 mm thick, TE/TR=30/1500 msec, SMS acceleration factor 3, GRAPPA in-plane acceleration factor 2, no partial Fourier, 2315 Hz/Pixel bandwidth, 100 measurements) 10,11 on an fBIRN phantom12.To generate spike noise, two 30 AWG tinned copper wires were prepared with the ends of each exposed. One end of each wire was taped to the surface of the phantom such that the wires were in electrical contact. The other ends were secured at the end of the table. The wires were three meters in length to ensure they were long enough to go from the bore to the end of the patient table. Five datasets were generated. About 20% of the way through each acquisition, the two ends of the wire located at the end of the table were quickly flicked together.
Results
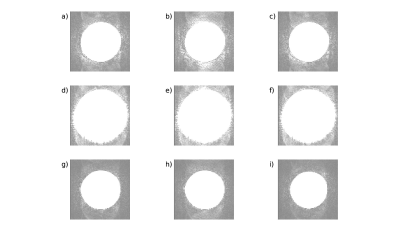
Artifacts were produced in each run. In Table 1, we show the total number of images afflicted by spike noise artifacts and the particular slices afflicted by the artifacts. Though some variability exists in the total number and location of slices affected, each run produced spike artifacts that were accurately detected by an SMS spike detection algorithm13 developed by the authors of this study.In Figure 2, we show 3 simultaneously acquired slices showing spike subtle artifacts as indicated by the arrows. Each row represents the same slice measured in three consecutive time points with the middle time point being afflicted by spike artifacts.
Discussion and Conclusion
Even faint spikes can corrupt sensitive measurements such as fMRI and ASL. An interesting aspect of this study was how difficult it was to generate faint spikes. Large, obvious artifact could be generated easily by simply moving a wire near the phantom. It was necessary to secure the wires to limit the extent of the artifact. The results are generally important in daily operation. Metallic objects left in the room in hidden places over ceiling tiles or under a raised floor (figure 3) have the potential to touch at random times and produce spike artifacts.Our methods may also be useful for machine learning approaches for artifact removal, as it is relatively straightforward to generate a large number of spikes for training a convolutional neural network (CNN)14,15,16.
In conclusion, we describe a simple procedure to generate spikes in images. Our methods are reproducible and simple to implement and may be useful for anyone who wants to produce reference data for algorithms designed to detect and eliminate spikes.
Acknowledgements
No acknowledgement found.References
[1] Xiaodong Zhang, Pierre-Francois Van De Moortele, Josef Pfeuffer, and Xiaoping Hu. Elimination of k-space spikes in fMRI data. Magnetic Resonance Imaging, 19(7):1037–1041, September 2001.
[2] S. Chavez, P. Storey, and S.J. Graham. Robust correction of spike noise: Application to diffusion tensor imaging. Magnetic Resonance in Medicine, 62(2):510–519, August 2009.
[3] M. Staemmler and K. Gersonde. Echo shape analysis and image size adjustment on the level of echoes: Improvement of parameter-selective proton images. Magnetic Resonance in Medicine, 3(3):418–424, June 1986.
[4] Spraggins T. Spike removal from MR raw data sets. Proceedings of the 6th Annual Meeting of ISMRM, New York, New York, USA., Abstract 789, 1987.
[5] T.K.F. Foo, N.S. Grigsby, J.D. Mitchell, and B.E. Slayman. SNORE: spike noise removal and detection. IEEE Transactions on Medical Imaging, 13(1):133–136, March 1994.
[6] J. W. Goldfarb. F. Schmitt. A robust method to remove spike artifacts through HPF postprocessing. Proceedings of the 4th Annual Meeting of ISMRM, New York, New York, USA., Abstract 1646, 1996.
[7] Yi-Hsuan Kao and J.R. MacFall. Correction of MR k-space data corrupted by spike noise. IEEE Transactions on Medical Imaging, 19(7):671–680, July 2000.
[8] Campbell A. E., Josephs O., Lythgoe M. F., Ordidge R. J., and Thomas D. L. A semi-automatic k-space despiking algorithm for the removal of striping artefact in mri images. Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia., Abstract 2443, 2012.
[9] Adrienne E. Campbell-Washburn, David Atkinson, Zoltan Nagy, Rachel W. Chan, Oliver Josephs, Mark F. Lythgoe, Roger J. Ordidge, and David L. Thomas. Using the robust principal component analysis algorithm to remove RF spike artifacts from MR images. Magnetic Resonance in Medicine, 75(6):2517–2525, July 2015.
[10] Steen Moeller, Essa Yacoub, Cheryl A. Olman, Edward Auerbach, John Strupp, Noam Harel, and Kâmil Uğurbil. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magnetic Resonance in Medicine, 63(5):1144–1153, April 2010.
[11] K. Setsompop, J. Cohen-Adad, B.A. Gagoski, T. Raij, A. Yendiki, B. Keil, V.J. Wedeen, and L.L. Wald. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. NeuroImage, 63(1):569–580, October 2012.
[12] Lee Friedman and Gary H. Glover. Report on a multicenter fMRI quality assurance protocol. Journal of Magnetic Resonance Imaging, 23(6):827–839, 2006.
[13] Sidhu et al (under review).
[14] Gurbani, SS, Schreibmann, E, Maudsley, AA, et al. A convolutional neural network to filter artifacts in spectroscopic MRI. Magn. Reson. Med. 2018; 80: 1765– 1775. https://doi.org/10.1002/mrm.27166
[15] Kyathanahally, S.P., Döring, A. and Kreis, R. (2018), Deep learning approaches for detection and removal of ghosting artifacts in MR spectroscopy. Magn. Reson. Med, 80: 851-863. https://doi.org/10.1002/mrm.27096
[16] Küstner, T., Liebgott, A., Mauch, L. et al. Automated reference-free detection of motion artifacts in magnetic resonance images. Magn Reson Mater Phy 31, 243–256 (2018). https://doi.org/10.1007/s10334-017-0650-z
Figures