5014
Motion-Resolved 4D Radial Ultrashort Echo Time (UTE) Lung MRI with Built-in Camera-Based Respiratory Motion Sensing1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Philips Healthcare, MR R&D, Rochester, MN, United States, 3Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Camera-based respiratory motion sensing (VitalEye) was successfully used to compute a respiratory signal for motion-resolved 4D radial ultrashort echo time (UTE) lung MRI. k-space data were sorted with respect the respiratory signal and binned into 10 different motion states to resolve respiratory motion. The respiratory signals from VitalEye were comparable to self-navigators. The 4D lung MRI reconstructions from both VitalEye and self-navigators were able to resolve respiratory motion and the signal intensity profiles along with the lung-liver interface and pulmonary vessels demonstrated that they both provided sharper image contrast compared to the motion-averaged images.
INTRODUCTION
Respiratory motion remains a significant challenge for lung MRI. Breath holds and respiratory gating are standard techniques to compensate for respiratory motion, but they are patient dependent or inefficient and usually result in 3D imaging with poor performance.1,2 3D radial acquisitions are often more motion tolerant than Cartesian sequence, and motion artifacts have a more benign appearance. In addition to motion robustness, center-out radial ultrashort echo time (UTE) is attractive in lung MRI due to the short T2* of the lung parenchyma. Motion-resolved lung imaging has been proposed to further reduce image blurring, where k-space data were sorted and binned into different motion states using respiratory signals from self-navigators or bellows.3-6 Recent studies have shown that camera-based respiratory motion compensation provided comparable or better image quality in cardiac and abdominal imaging compared to a conventional belt or navigator techniques.7,8 In addition, a VASP sequence was developed to reduce scan time or aliasing artifacts in 3D radial imaging.9 The purpose of this study was to develop motion-resolved 4D radial UTE lung MRI using VASP data acquisition and respiratory signals from camera-based respiratory sensing.METHODS
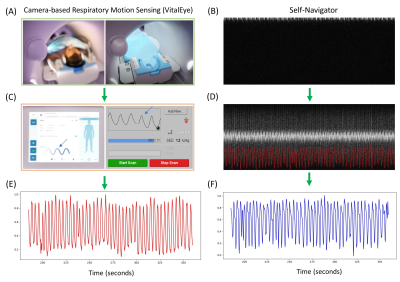
Respiratory Motion Signal: The VASP sequence was modified for 3D radial UTE lung MRI by introducing center-out half radial spokes. Figure 1 shows the workflow of getting respiratory signals from camera-based respiratory sensors (VitalEye, Philips Healthcare) and self-navigators. The camera is installed at the rear cover of the MRI scanner to acquire video data of the subject's torso. Real-time respiratory signal was automatically derived at a rate of 20 Hz and was streamed to the MRI data acquisition system and patient physiology interface.10,11 For self-navigators, a half radial spoke in the head-feet direction was acquired at the beginning of each interleave at an interval of 250 ms. The respiratory signal from self-navigators was then extracted by a fast Fourier transform of the multi-coil k-space data to generate projection profiles in the head-feet direction followed by a motion detection algorithm described previously.12VASP Data Acquisition: Free-breathing T1-weighted UTE VASP data acquisition was performed on two healthy volunteers (1 male, 1 female) on a clinical 3T MRI scanner (Ingenia Elition X, Philips Healthcare) using a combination of 16-channel anterior and 12-channel posterior coils with the following sequence parameters: TR/TE = 3.7/0.12 ms, flip angle = 5°, FOV = 300×300×300 mm3, voxel size = 1.1×1.1×1.1 mm3, spokes per interleave = 67, number of interleaves = 1547, bandwidth = 577 Hz/pixel, total scan time = 6:33 min. K-space data and their associated 3D coordinates, density compensation weights, and respiratory signals were exported from the scanner for subsequent offline 4D lung image reconstruction. The study was approved by local institutional review board and written informed consent was obtained from the subjects prior to MRI scans.
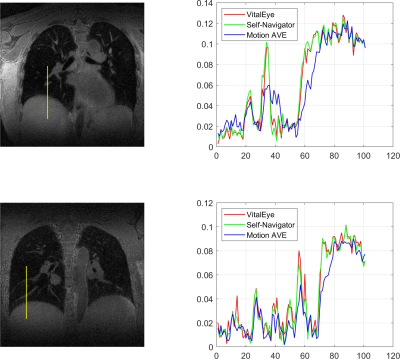
4D Image Reconstruction: Image reconstruction was performed offline using an inhouse pipeline in Python. Coil sensitivity maps were reconstructed from the complete k-space data using the JSENSE algorithm.13 k-space data were then sorted with respect to the respiratory signal and then binned into 10 different motion states from expiration to inspiration. Motion-resolved 4D lung image reconstruction was performed similar to the XD-GRASP framework.14 NUFFT operations were implemented using the SigPy package and the first-order finite difference was chosen as the sparsifying transform along the temporal domain.6,15 The reconstruction time of each 4D dataset was about two hours. The profiles of signal intensity along with the lung-liver interface and pulmonary vessels were calculated to evaluate the performance of 4D lung MRI using VitalEye respiratory signal for resolving motion compared to self-navigators.
RESULTS
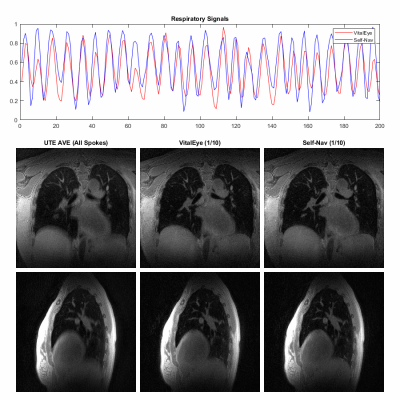
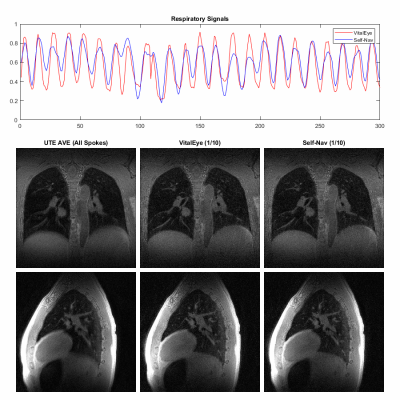
Data acquisition and image reconstruction were successfully performed for both subjects. Figures 2 and 3 show the results of the respiratory signals and corresponding 4D lung MRI reconstructions from the two healthy volunteers, respectively. There was a good agreement between the respiratory signals from VitalEye and self-navigators. Pulmonary vessels and lung-liver interface (diaphragm) can be well visualized on the motion-averaged images from gridding reconstruction of all k-space data despite some residual blurring, which was improved on motion-resolved 4D lung MRI reconstructions with both VitalEye and self-Navigators. The intensity profiles in Figure 4 further confirm that motion-resolved 4D MRI reconstructions from VitalEye and self-navigators provide comparable contrast along with the lung-liver interface and pulmonary vessels, and they show increased diaphragm and vessel sharpness compared to the motion-averaged images.DISCUSSION
The camera-based respiratory motion sensing method (VitalEye) provides reliable and high sampling rate of the respiratory motion signal in 4D lung MRI to resolve the respiratory motion. There is no need for additional patient setup. Therefore, VitalEye offers an alternative solution for motion-resolved 4D MRI and may become a preferred choice when a high sampling rate of respiratory motion is desired (such as in real-time motion tracking16) and in contrast-enhanced experiments where the respiratory signal from VitalEye is independent of contrast or signal intensity change. Further study is warranted to quantitatively compare the image quality and ability to resolve respiratory motion between VitalEye and self-navigators in a larger cohort of patients.CONCLUSION
The built-in camera-based respiratory motion sensing method (VitalEye) provides reliable respiratory signals comparable to self-navigators that can be used in 4D radial UTE lung MRI to resolve respiratory motion.Acknowledgements
None.References
1. Gai ND, Malayeri A, Agarwal H, et al. Evaluation of optimized breath-hold and free-breathing 3D ultrashort echo time contrast agent-free MRI of the human lung. J Magn Reson Imaging 2016;43(5):1230-1238.
2. Tokuda J, Morikawa S, Haque HA, et al. Adaptive 4D MR Imaging Using Navigator-Based Respiratory Signal for MRI-Guided Therapy. Magn Reson Med 2008;59(5):1051-1061.
3. Jiang W, Ong F, Johnson KM. et al. Motion robust high resolution 3D free-breathing pulmonary MRI using dynamic 3D image self-navigator. Magn Reson Med 2018;79(6):3954-2967.
4. Zhu X, Chan M, Lustig M, et al. Iterative motion-compensation reconstruction ultra-short TE (iMoCo UTE) for high-resolution free-breathing pulmonary MRI. Magn Reson Med. 2020;83(4):1208-1221.
5. Feng L, Delacoste J, Smith D, et al. Simultaneous Evaluation of Lung Anatomy and Ventilation Using 4D Respiratory-Motion-Resolved Ultrashort Echo Time Sparse MRI. J Magn Reson Imaging 2019;49(2):411-422.
6. Ong F, Zhu X, Cheng JY, et al. Extreme MRI: Large-scale volumetric dynamic imaging from continuous non-gated acquisitions. Magn Reson Med 2020;84(4):1763-1780.
7. Harder F, Lohofer FK, Kaissis GA, et al. Camera-based respiratory triggering improves the image quality of 3D magnetic resonance cholangiopancreatography. Eur J Radiol 2019;120(1):108675.
8. Gottwald LM, Blanken CP, Tourais J, et al. Retrospective Camera-Based Respiratory Gating in Clinical Whole-Heart 4D Flow MRI. J Magn Reson Imaging 2021;54(2):440-451.
9. Krishnamoorthy G, Smink J, Tourais J, et al. Variable anisotropic FOV for 3D radial imaging with spiral phyllotaxis (VASP). Magn Reson Med 2020;85(1):68-77.
10. Rocque M. Fully automated contactless respiration monitoring using a camera. 2016 IEEE International Conference on Consumer Electronics: IEEE; 2016. p 478-479.
11. Kruger MG, Springer RPW, Kersten GM, et al. Contact-less Vital Sign Monitoring using a COTS Resource-Constrained Multi-Core System. IECON 2019 – 45th Annual Conference of the IEEE Industrial Electronics Society: IEEE; 2019. p 3057-3062.
12. Feng L, Huang C, Shanbhogue K, et al. RACER-GRASP: Respiratory-weighted, aortic contrast enhancement-guided and coil-unstreaking golden-angle radial sparse MRI. Magn Reson Med 2018;80(1):77-89.
13. Ying L, Sheng J. Joint image reconstruction and sensitivity estimation in SENSE (JSENSE). Magn Reson Med 2007;57(6):1196-1202.
14. Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med 2016;75(2):775-788.
15. Ong F, Lustig M. SigPy: a python package for high performance iterative reconstruction. Proc Int Soc Magn Reson Med, 2019, 27:4819.
16. Feng L, Tyagi N, Otazo R. MRSIGMA: Magnetic Resonance SIGnature MAtching for real-time volumetric imaging. Magn Reson Med 2020;84(3):1280-1292.
Figures