4994
Prospective Motion Correction for MR Spectroscopy in the Human Brain Using Multi-Slice Spiral Navigator1University of Minnesota, MINNEAPOLIS, MN, United States
Synopsis
MR spectroscopy is prone to motion artifacts due to long scan times. Existing motion correction techniques require additional hardware or are inherently slow. We demonstrate a fast and accurate prospective motion correction method for MRS using spiral navigators. A multi-slice to volume registration approach accelerates the motion parameter determination and achieves artifact free spectra at intended volume of interest.
Introduction
Magnetic resonance spectroscopy (MRS) is an invaluable tool in probing metabolites in vivo. However, subject motion is a major concern especially in pediatric, elderly, and patients with movement disorders. To have reasonable spectral signal-to-noise (SNR) for reliable metabolites quantification, the MRS scan time is typically long (>5 min). This makes MRS more susceptible to motion and hardware instabilities. The subject motion will degrade the spectral quality, i.e. insufficient water suppression, broad spectral linewidth, reduced SNR, and can lead to lipid contamination. In addition, motion can misplace the MRS volume-of-interest (VOI) leading to poor diagnosis.Several prospective motion correction techniques currently exist in the brain and use either external tracking devices e.g., optical camera1 and NMR probes2, or are image-based navigators e.g. spiral3, volumetric EPI4, or FID5. External tracking devices are expensive due to the cost of the additional hardware and software requirement and the need to have these integrated with the MR scanner6. On the other hand, prospective navigators can be implemented into a custom MRS sequence and do not require additional hardware. Studies that used image-based navigators have a relatively long time between measurement of motion and the acquisition of the MRS data (≥1 s)3-4. Any motion occurring during that time cannot be corrected.
Therefore, the aim of the current study was to develop and implement a fast image-based motion tracking method while minimizing the time between the start of the motion navigator and the start of the MRS acquisition. This fast navigator was based on 2D spiral imaging with multi-slice-to-volume registration7 for rapid computation of motion parameters.
Methods
A motion navigator was implemented based on spiral imaging for efficient coverage of k-space in single-shot. We choose the spiral-IN/OUT trajectory8 to minimize off-resonance effects near the sinus cavities. At the start of the study, a reference volume was acquired (TR/TE =75/15ms, FOV=192x192 mm2, matrix=64x64, 30 slices, thk=3 mm). During the MRS acquisition, the navigator consisted of 3 slices (chosen to not overlap with the MRS voxel) and was executed during each TR. Motion parameters (3 translations and 3 rotations) were then estimated by multi-slice-to-volume image registration using the Insight tool kit (ITK) library available on the scanner.The sLASER sequence was modified to incorporate a real-time image-based spiral navigator, as well as a real-time shim and frequency corrections as described in our previous work9. Figure 1 shows a schematic of the acquisitions performed during the study.
All experiments were performed on a 3T MR system (MAGNETOM Prisma, Siemens Healthcare) with a 20-channel head coil. The study was approved by the institutional review board and written informed consent was obtained from the participating healthy subject. The data acquisition protocol started with obtaining a 2D spiral reference volume. 3D MPRAGE images were then acquired to position an 8 mL VOI in the prefrontal cortex. Single-voxel sLASER data (TR/TE =5000/28ms, 32 averages) was obtained where water suppression was achieved using the metabolite cycling technique.
Three configurations were tested: (i) without subject motion, (ii) with subject motion and motion correction (MoCo) disabled, (iii) with subject motion and MoCo enabled. A pre-determined head motion protocol was described to the subject and they were asked to tilt their head slowly to the left, raise their chin up, and return to their original position based on the operators’ oral instructions during the MRS measurements. In all acquisitions, shim and frequency navigators were enabled. The motion was also tracked using an optical camera to validate the image-based navigator data.
Results
High-quality spiral reference images were acquired with no obvious blurring artifacts (Figure 2A). An example of the navigator image acquired inside the MRS sequence is shown in Figure 2B when the subject moved their head and the corrected image. The total time to acquire the three spiral navigator images including registration and feedback was ~370 ms.Figure 3 shows sLASER spectra and the associated motion plots acquired from one subject with the 3 configurations (no motion, motion with/without MoCo). Comparable high-quality spectra were observed when the subject did not move or when MoCo was enabled. With MoCo disabled, the intended voxel was at the wrong location, as demonstrated by the difference in total creatine and total choline ratio (Figure 3b).
Discussion and Conclusion
We have demonstrated the feasibility of prospective motion correction in MRS using fast spiral navigators. The observed contrast changes between the reference and multi-slice navigator images are most likely due to the change in linear shims and change in magnetization due to OVS and metabolite-cycling pulses. To avoid disturbing the magnetization during MRS data acquisition, the navigator slices were placed outside of the MRS VOI (green dotted lines in Figure 3).There are several ways to further reduce the total time between motion navigator and MRS acquisitions: use spiral-IN technique with B0 offset correction instead of spiral-IN/OUT; accelerate the image registration using 16 multi-threads, instead of 8 currently implemented; and only use the frequency navigator module when the frequency offset from dynamic shim is above a certain threshold. This study shows the feasibility to correct for motion with minimal time between navigator and MRS acquisition.
Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH) R01 EB030000, P41 EB027061, and P30 NS076408. We thank Daniel Hoinkiss for his help with the ITK image registration implementation.
References
1. Zaitsev, M., Speck, O., Hennig, J. and Büchert, M. (2010), Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR Biomed., 23: 325-332. https://doi.org/10.1002/nbm.1469
2. Haeberlin, M., Kasper, L., Barmet, C., Brunner, D.O., Dietrich, B.E., Gross, S., Wilm, B.J., Kozerke, S. and Pruessmann, K.P. (2015), Real-time motion correction using gradient tones and head-mounted NMR field probes. Magn. Reson. Med., 74: 647-660. https://doi.org/10.1002/mrm.25432
3. Keating B, Deng W, Roddey JC, et al. Prospective motion correction for single-voxel 1H MR spectroscopy. Magn Reson Med. 2010;64(3):672-679. doi:10.1002/mrm.224484 Hess et al. Magn Reson Med 2011.
4. Hess, A.T., Dylan Tisdall, M., Andronesi, O.C., Meintjes, E.M. and van der Kouwe, A.J.W. (2011), Real-time motion and B0 corrected single voxel spectroscopy using volumetric navigators. Magn. Reson. Med., 66: 314-323. https://doi.org/10.1002/mrm.22805
5. Waszak, Maryna, et al. "Prospective head motion correction using FID‐guided on‐demand image navigators." Magnetic resonance in medicine 78.1 (2017): 193-203.6 , , , et al. Motion correction methods for MRS: experts' consensus recommendations. NMR in Biomedicine. 2021; 34:e4364. https://doi.org/10.1002/nbm.4364
7. Hoinkiss DC, Porter DA. Prospective motion correction in 2D multishot MRI using EPI navigators and multislice-to-volume image registration. Magn Reson Med. 2017 Dec;78(6):2127-2135. doi: 10.1002/mrm.26951. Epub 2017 Oct 5. PMID: 28983957.
8. Fielden SW, Meyer CH. A simple acquisition strategy to avoid off-resonance blurring in spiral imaging with redundant spiral-in/out k-space trajectories. Magn Reson Med. 2015 Feb;73(2):704-10. doi: 10.1002/mrm.25172. Epub 2014 Mar 6. PMID: 24604539; PMCID: PMC4156933.
9. Deelchand DK, Joers JM, Auerbach EJ, Henry PG. Prospective motion and B0 shim correction for MR spectroscopy in human brain at 7T. Magn Reson Med. 2019;82(6):1984-1992. doi:10.1002/mrm.27886
Figures
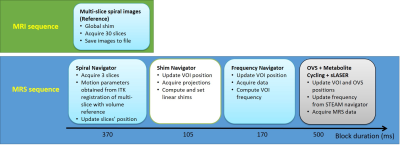
Figure 1. Schematic of the navigator-based motion correction employed in the study. Block duration includes the feedback time between the reconstruction machine and the scanner.
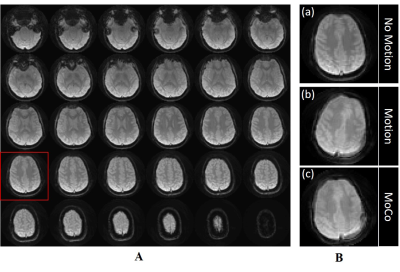
Figure 2. Prospective motion correction results obtained with 2D spiral navigators. (A) represents the 30 slice reference volume data acquired at the start of the study. (B) represents 1 of the 3 navigator slices obtained during MRS acquisition after subject moved their head. (c) represents motion correction navigator of (b) using motion parameters obtained after ITK image registration.
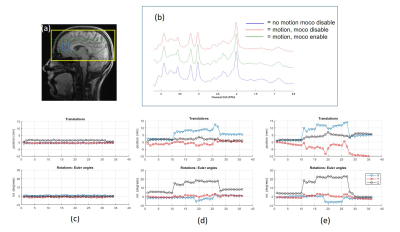
Figure 3. Prospective motion corrected MRS. (a) Shows the 3D reference with the yellow box representing the spiral volume reference, the blue box representing the MRS VOI, and green lines representing the 3-slice spiral navigator used with this voxel (ref figure 2). (b) Shows the reference spectra (in blue), spectra without motion correction (in red), and spectra with motion correction (in green). (c-e) represents the translation and rotation parameters for no motion, motion with MoCo disabled and motion with MoCo enabled.