4980
Abdominal DCE-MRI in mice with stack of stars sampling and KWIC image reconstruction1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Dynamic contrast enhanced MRI data in the abdomens of small animal models is often corrupted due to the effects respiratory and peristaltic motion. Here a DCE protocol that employs stack of stars sampling throughout was implemented and was shown to be robust with respect to motion artifacts. The sampling scheme also facilitates image reconstruction methods that employ view sharing, notably KWIC, yielding images with high temporal and spatial resolution. The protocol was demonstrated in an orthotopic murine model of pancreatic cancer. The resulting data were analyzed using the reference tissue method and provided high quality Ktrans and ve parameter maps.
INTRODUCTION
Application of DCE methods to pre-clinical models of orthotopic tumors of the abdomen is limited due to artifacts generated by rapid respiratory motion. Here we demonstrate a DCE protocol that employs stack of stars (SOS) sampling techniques in order to minimize motion artifacts. SOS sampling uses radial sampling in two dimensions with phase encoding in the third thereby providing over sampling of the center of k-space resulting in reduced motion artifacts. The sampling scheme also provides opportunities for application of view sharing image reconstruction techniques for improved temporal resolution. The technique is demonstrated in a mouse model of pancreatic cancer.METHODS
The proposed technique was demonstrated in a genetically engineered mouse model of pancreatic ductal adenocarcinoma (1) that spontaneously develops tumors at age 17–19 weeks. Tumor bearing animals (n=21) were prepared for MRI exams by placement of a tail vein catheter, induction of general anesthesia and placement of temperature and respiration probes. Core body temperature was maintained at 37° during the exam by directing a regulated warm air source over the animal. All protocols in the DCE study used identical FOV parameters and a spoiled GRE acquisition with golden angle (2) SOS sampling (3, 4). Following coil positioning, scanner calibration and generation of scout images, B1 mapping was performed using the Actual Flip-angle Imaging (AFI) (5) method (TE/TR1/TR2 = 1.2/13/65 msec, flip angle = 60°, matrix = 128 x 201 x 16 (read x views x phase), FOV = 32 x 32 x 24 mm3, averages = 2, total acquisition time = 8.4 min). This was followed by acquisition of Variable Flip Angle (VFA) T1 mapping (6, 7) data (TR/TE=5.5/1.25 msec, flip angles = 2, 5, 8, 12, 16, 20°, averages = 4, total acquisition time ~ 7 min) and acquisition of the DCE series (TR/TE=5.5/1.25 msec, flip angle = 9°, averages = 1, repetitions = 40, total acquisition time = 11.7 min). Baseline data were acquired for 2 min following the start of the dynamic scan, at which time 0.2 ml of 10 mM Gd contrast agent was injected via the tail vein catheter.The B1 and T1 data were reconstructed to 128 x 128 images using standard regridding methods (8). The B1 maps were generated as described elsewhere (5) and used to correct flip angles in the processing of the subsequent scans. Baseline T1 maps were generated via least squares fitting of the VFA signal intensity to the Ernst equation. The dynamic data were reconstructed using the KWIC method of view sharing (9) resulting in an effective temporal resolution of 4.3 sec. Pixel-wise quantitative analysis of the DCE time series was performed using the reference tissue method (10, 11) with muscle serving as the reference tissue yielding maps of Ktrans and ve. Regions of interest (ROI) for tumor, kidney cortex and erector spinae (back) muscle were manually drawn and median values of the perfusion parameters over the ROIs were tabulated.
RESULTS
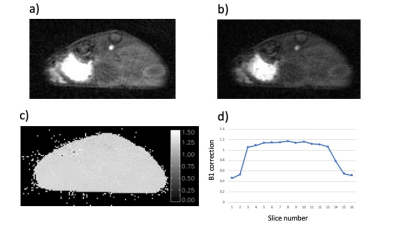
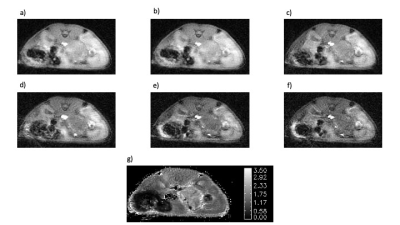
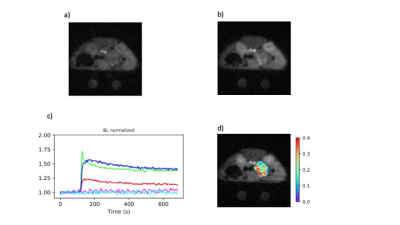
The images generated by the AFI protocol were free from motion artifacts and had SNR in the range of 7-15. The calculated maps of B1 were very flat within each slice and reflected the excitation pulse profile in the axial dimension (Fig-1). Variations in mean B1 amplitude between studies were evident suggesting day to day variations in the accuracy of the RF calibration of approximately 10%. The VFA images were also of high SNR and free from motion artifacts (Fig-2). Inclusion of the B1 maps in the analysis of the VFA data reduced the variability in tissue T1 estimates relative to maps generated without the correction. T1 values of various tissue were reproducible with median values averaged over all animals of tumor/kidney/muscle =2.42±0.52/1.76±0.42/1.99±0.41 sec. The sampling scheme and view sharing techniques employed in the dynamic studies resulted in artifact free images with high temporal and spatial resolution (Fig-3) and a SNR range of 6-16 for the tissues of interest. Time series plots of mean signal intensity over the various ROIs had high SNR and clearly reflected the tracer dynamics. The reference tissue model provided high resolution parameter maps with median values over the ROIs averaged over all animals of Ktrans tumor/kidney = 0.20±0.14/0.75±0.20 min-1 and ve = 0.28±0.17/0.33±0.13. The standard deviation in the Ktrans values reflects inter-animal variability (median range = 0.08-0.48) as well as intra-tumor heterogeneity. Typically tumors had elevated Ktrans values around the periphery of the tumor relative to the core.DISCUSSION
Rapid respiration rates in small animal models typically result in severe motion artifacts for DCE MRI studies of orthotopic tumors in the abdomen. Respiratory gating cannot be used to suppress these artifacts in dynamic studies because it is incompatible with the temporal resolution requirements of the method. Here we demonstrate that golden angle SOS sampling combined with view shared image reconstruction techniques can be applied in abdominal DCE studies to generated artifact free images with high temporal resolution.CONCLUSION
The combination of golden angle SOS sampling with view shared image reconstruction techniques yields high quality spoiled GRE dynamic images in pre-clinical abdominal DCE studies. Analysis of the resulting data via the reference tissue method provides high resolution maps of perfusion parameters.Acknowledgements
All MRI studies were performed in the Small Animal Imaging Facility in the University of Pennsylvania Department of Radiology. Funding support from NIH : U24-CA-231858, P30-CA-016520.References
1. Hingorani, S. R.; Petricoin, E. F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M. A.; Ross, S.; Conrads, T. P.; Veenstra, T. D.; Hitt, B. A.; Kawaguchi, Y.; Johann, D.; Liotta, L. A.; Crawford, H. C.; Putt, M. E.; Jacks, T.; Wright, C. V. E.; Hruban, R. H.; Lowy, A. M.; Tuveson, D. A., Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse (vol 4, pg 434, 2003). Cancer Cell 2004, 5 (1), 103-103.
2. Winkelmann, S.; Schaeffter, T.; Koehler, T.; Eggers, H.; Doessel, O., An optimal radial profile order based on the golden ratio for time-resolved MRI. Ieee Transactions on Medical Imaging 2007, 26 (1), 68-76.
3. Peters, D. C.; Korosec, F. R.; Grist, T. M.; Block, W. F.; Holden, J. E.; Vigen, K. K.; Mistretta, C. A., Undersampled projection reconstruction applied to MR angiography. Magnetic Resonance in Medicine 2000, 43 (1), 91-101.
4. Song, H. K.; Dougherty, L., k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magnetic Resonance in Medicine 2000, 44 (6), 825-832.
5. Yarnykh, V. L., Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magnetic Resonance in Medicine 2007, 57 (1), 192-200.
6. Fram, E. K.; Herfkens, R. J.; Johnson, G. A.; Glover, G. H.; Karis, J. P.; Shimakawa, A.; Perkins, T. G.; Pelc, N. J., RAPID CALCULATION OF T1 USING VARIABLE FLIP ANGLE GRADIENT REFOCUSED IMAGING. Magnetic Resonance Imaging 1987, 5 (3), 201-208.
7. Cheng, H. L. M.; Wright, G. A., Rapid high-resolution T-1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magnetic Resonance in Medicine 2006, 55 (3), 566-574.
8. Sullivan, J. D. O., A Fast Sinc Function Gridding Algorithm for Fourier Inversion in Computer Tomography. IEEE Transactions on Medical Imaging 1985, 4 (4), 200-207.
9. Song, H. K.; Dougherty, L., Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magnetic Resonance in Medicine 2004, 52 (4), 815-824.
10. Yankeelov, T. E.; Luci, J. J.; Lepage, M.; Li, R.; Debusk, L.; Lin, P. C.; Price, R. R.; Gore, J. C., Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magnetic Resonance Imaging 2005, 23 (4), 519-529.
11. Jones, K. M.; Pagel, M. D.; Cardenas-Rodriguez, J., Linearization improves the repeatability of quantitative dynamic contrast enhanced MRI. Magnetic Resonance Imaging 2018, 47, 16-24.
Figures