4977
Spectrally encoded multi-spectral imaging (SEMSI) for off-resonance correction near metal with multi-spin echo and parallel imaging1Bioengineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Metal implants induce severe B0 field inhomogeneities, causing severe distortion near the implant. In this work, we combined multi-echo spin echo and parallel imaging to an approach that uses echo shifting to encode spectral components and resolve the metal-induced artifacts. Phantom and in vivo experiments were conducted and indicated that the proposed method provides comparable artifact reduction and image quality to existing multispectral imaging approaches in comparable scan time.
Introduction
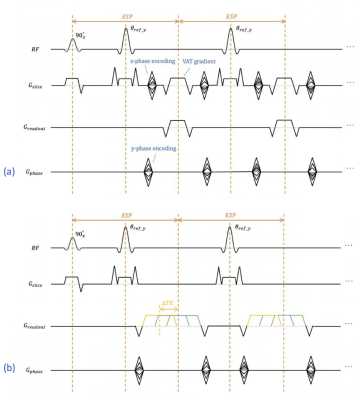
Metal implants induce severe B0 field inhomogeneities that create strong, static background gradients. These background gradients result in distorted slice profiles, creating through-plane artifacts, and cause severe readout shifts. Conventional multispectral imaging (MSI) approaches such as SEMAC [1] and MAVRIC-SL [2] apply z-phase encoding to resolve through-plane distortion and view-angle tilting (VAT) to reduce in-plane distortion. We present an alternate approach to resolving metal-induced artifacts based on the previously presented SEMSI sequence [3], with the addition of GRAPPA in y-ΔTE space. SEMSI uses echo shifting to separate excited spectral components (Fig.1), which offers a simpler encoding approach to conventional MSI that avoids global blurring caused by VAT.Theory
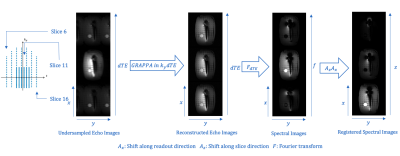
Due to metal-induced field inhomogeneity, slice-selective excitation will excite spins outside the principal slice. Like N-point spectroscopy, which uses echo shifting to create phase differences, spectral components in metal images can be separated by acquiring multiple echo shifts. Each echo image is a xy-image projected along the slice dimension, but with different phase accruals from off-resonance that also depend on z location. Spectral components within each bin can be separated by performing a 1D Fourier transform along ΔTE dimension. Slice and readout direction shifts are applied to the spectral images to restore their original location using the off-resonance frequency and gradient amplitudes. Spectral images are combined with root sum of squares. The reconstruction pipeline is shown in Figure 2.Methods
Acquisitions were performed at 3T (Signa Premier, GE Healthcare). SEMSI, SEMAC and 2D Fast-Spin Echo (FSE) were used to scan a shoulder replacement embedded in agar. Sequence parameters were: TE 8 ms, TR 3000 ms, acquisition matrix 256 x 256, ETL 20, slice thickness 2.5 mm, 20 bins, RF bandwidth 1 kHz, readout bandwidth ±125 kHz. Echo time shifts for SEMSI ranged from –520 us to 468 us with 52 us increments. Data was both retrospectively and prospectively 2x2 undersampled in ky-ΔTE space with a 16 x 12 calibration region, 0.6 partial Fourier along ky. GRAPPA [4] was used to interpolate missing ky-kz lines for SEMAC and missing ky-ΔTE lines for SEMSI. Homodyne was used for partial Fourier correction.Following interpolation of missing lines, spectral components in each bin were separated by 1D Fourier transform along ΔTE dimension. The frequency for each spectral component is $$$ Δf = n * 1/ (520+468) us $$$, where n is the index of the corresponding echo shift. Shifts along the readout and slice directions were calculated by Δf /readout_bandwidth and Δf /rf_bandwidth respectively and performed by applying linear phase in the spatial Fourier transform space.
Following IRB approval and informed consent, sequences were applied in a volunteer with two titanium screws from ACL reconstruction surgery, 16-channel flex coil. Parameters were: TE 8 ms, TR 3000 ms, acquisition matrix 256 x 256, ETL 24, slice thickness 4 mm, 20 bins. RF bandwidth 1 kHz, readout bandwidth ±125 kHz, 2x2 uniform undersampling, partial Fourier 0.6 Fully sampled 2D FSE was acquired for comparison.
Results
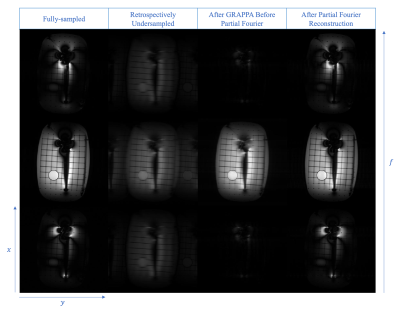
Figure 3 shows the retrospective undersampling results. Root mean square error of the reconstructed spectral images is 4.26%.Figure 4 shows the phantom results. Metal-induced artifacts, including signal-loss and pile-up that exist on the 2D FSE images, are suppressed on SEMSI and SEMAC images. However, ringing artifacts showed around the metal implant on both images.
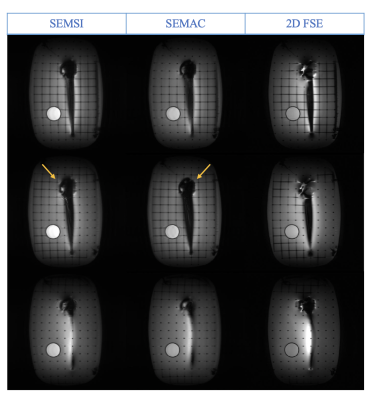
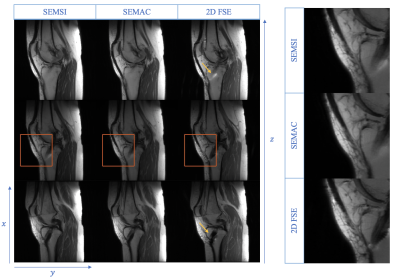
In vivo results are shown in Figure 5. Pile-up artifacts present on 2D FSE were reduced using SEMSI and SEMAC. Column on the right shows the zoomed images of the anterior knee region, which shows that the SEMSI and SEMAC images have comparable resolution to the fully sampled 2D FSE images.
Discussion
In our experiment, reconstruction for partial Fourier sampling was performed in ky-f space instead of independently for each echo image in kx-ky space to maintain the phase difference created by the echo shifts. Homodyne reconstruction of each echo image requires phase correction to change the images to real-valued thus causing a failure in later step of separating spectral components by Fourier transform.In SEMSI, spectral components are found by applying the Fourier transform in the ΔTE dimension and registered by linear phase. ΔTE is the transform domain of the slice location z, with a phase difference corresponding to off-resonance frequency. Thus, ΔTE can be viewed as kz in 3D imaging with extra phase. Though k-space interpolation was performed in ky-f space before registration using linear phase, GRAPPA can instead be performed after the slice registration step. The GRAPPA kernels estimated after registration have the same magnitude as those estimated before registration but with additional linear phase.
Ringing near the head of the implants is present in both SEMSI and SEMAC phantom images. Ringing in SEMAC is caused by insufficient slice selection or readout gradients. For SEMSI, these may be caused by low resolution in the frequency dimension, which results in frequency components not being registered to the correct slice location. This could be improved by adding additional gradient echoes at no significant scan time cost.
Conclusion
Off-resonance artifacts from metal can be encoded with echo shifting. Parallel imaging can be applied to produce clinically feasible scan times.Acknowledgements
This work was supported by R01 AR078912 and R01 EB017739.References
[1] Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice encoding for metal artifact correction in MRI. Magn Reson Med. 2009;62(1):66-76.
[2] Koch KM, Brau AC, Chen W, et al. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011;65(1):71-82.
[3] Yoon D, Lee PK, Nayak KS, Hargreaves BA, Spectrally-encoded Multi-spectral Imaging (SEMSI) for Off-resonance Correction Near Metallic Implants. ISMRM. May 2021, Vancouver.
[4] Griswold MA, Jakob PM, Heidemann RM, et al. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210.
[5] Ma J, Singh SK, Kumar AJ, Leeds NE, Broemeling LD. Method for efficient fast spin echo Dixon imaging. Magn Reson Med. 2002;48(6):1021-1027.
Figures