4969
Phase Correction Approach for Receive-Only Frequency Translation Studies1Electrical and Computer Engineering, Texas A&M University, College Station, TX, United States, 2Biomedical Engineering, Texas A&M University, College Station, TX, United States
Synopsis
23Na and 31P spectroscopy are powerful tools in assessing muscles in Duchenne Muscular Dystrophy studies. Frequency translation has been previously introduced as a means to facilitate multichannel studies on systems equipped with only 1H receiver arrays. Many approaches to frequency translation require mixing on both transmit and receive, which bypasses the need for phase correction. Presented here is an approach to receive-only translation of acquired data to the 1H frequency, that allows for post-processing phase correction using signals coupled from the host system’s and translator’s local oscillators.
Introduction
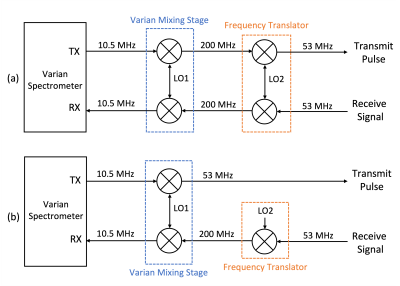
Multiple 31P biomarkers have been shown to be useful in assessing muscles with DMD in spectroscopy studies [1,2,3]. Recent studies have investigated 23Na as an early sensitive indicator of response to treatment in DMD patients [4,5]. Due to the lower abundance and lower gyromagnetic ratio of 31P and 23Na, it is beneficial to use array coils and perform averaging for improving SNR [6]. Frequency translation techniques have been reported previously in order to utilize narrow-band 1H array receivers for acquisition of multi-channel X-nuclear data in various studies [7,8]. There are two general approaches to implement the frequency translator, as shown in Figure 1: Translating on both transmit path and receive path easily achieves phase stability, as the same local oscillator is used to convert down during transmit and to convert up during receive. Any phase shift introduced by the local oscillator frequency during down converting (or up converting) is canceled out by the same local oscillator frequency during up converting (or down converting). However, there are some notable disadvantages for this translation approach. The system transmit chain can have devices such as directional couplers and circulators that are frequency specific, and mixing on transmit path may cause problems with built-in SAR monitoring expecting a different frequency. Therefore, one might find it beneficial to only mix on the receive path, as the scanner will still be operating in its default settings during transmit. In this case, phase stability is not automatically maintained by the hardware, due to the frequency change of the local oscillator in the scanner’s first mixing stage between transmit and receive, and the introduction of the additional translator local oscillator to only the receive path. In this study, we propose to translate only on receive path, and provide an approach to correct the phase shift over repetitions in post processing, by acquiring phase information from both the scanner’s first stage local oscillator and the translator’s oscillator. The advantages of this method are the compatibility with any X-nuclei frequency, and perhaps most importantly, as the phase is not collected from the detected signal, the method is applicable to low SNR studies.Method
The 31P and 23Na spectra were acquired on a 4.7T Varian scanner separately using single-tuned transceiver solenoid coils. A total of 36 average scans were taken for each nucleus, and each FID was saved individually. The scanner host computer is set to transmit at X-nuclear frequency and to receive at 1H frequency. The received signal was converted up to 1H frequency using one of the 16 channels in the frequency translator, presented previously [7]. The local oscillator for the translator was provided by an additional waveform generator from the Varian spectrometer, and was set to be the difference between the X-nuclear frequency and the 1H frequency. A high-speed digitizer was used to digitize the signals coupled from the scanner’s first stage local oscillator and the translator’s local oscillator. During transmit, the phase of the scanner’s first stage local oscillator was acquired. During receive, the phase of the scanner’s local oscillator was acquired again, as well as the phase of the local oscillator supplied to the frequency translator. A phase correction factor was derived using the phase information for each repetition, and was later applied to the spectrum corresponding to that repetition.Results
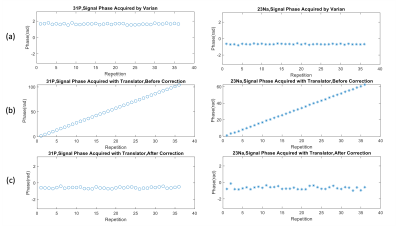
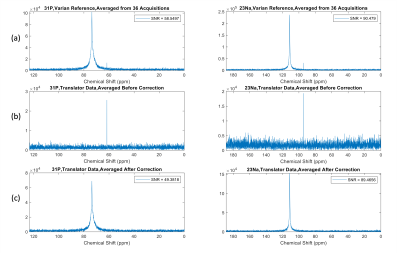
Figure 2 and Figure 3 show the phase of the signal and averaged spectra acquired by the stock Varian system, as well as the data acquired with the frequency translator implemented and averaged before and after applying the phase correction. Without phase correction, the data acquired with the frequency translator shows a significant phase ramp, and as a result, the signal cancels out during the averaging process. After applying phase correction, the averaged translator data shows improved SNR compared to the uncorrected data for both 31P and 23Na. The data shows the phase correction method presented facilitates significant improvement in phase stability from the receive-only translation as achieved by the Varian stock system.Discussion
In this study, we have provided an approach to implement the frequency translator by mixing on receive path only on a 4.7T Varian scanner. The phase stability is maintained by performing straightforward phase correction in post processing. This approach requires only the ability to sample a coupled signal from the scanner and translator’s local oscillators, but does not require significant hardware modification to the host system. It is also compatible with low SNR studies, as the phase correction factor is derived from phase information in the local oscillators, but not the phase information of individual FID in each acquisition.Acknowledgements
This research was funded by the NIH grant RO1EB028533.References
[1] D. P. Younkin, P. Berman, J. Sladky, C. Chee, W. Bank, and B. Chance, "31P NMR studies in Duchenne muscular dystrophy," Age‐related metabolic changes, vol. 37, no. 1, pp. 165-165, 1987, doi: 10.1212/wnl.37.1.165.
[2] C. Wary, T. Naulet, J.-L. Thibaud, A. Monnet, S. Blot, and P. G. Carlier, "Splitting of Pi and other 31P NMR anomalies of skeletal muscle metabolites in canine muscular dystrophy," NMR in Biomedicine, vol. 25, no. 10, pp. 1160-1169, 2012, doi: 10.1002/nbm.2785.
[3] J. Dunn, I. Tracey, and G. K. Radda, "Exercise metabolism in Duchenne muscular dystrophy: a biochemical and [31P]-nuclear magnetic resonance study of mdx mice," Proceedings of the Royal Society of London. Series B: Biological Sciences, vol. 251, no. 1332, pp. 201-206, 1993.
[4] M. A. Weber, A. M. Nagel, K. Jurkat-Rott, and F. Lehmann-Horn, "Sodium (23Na) MRI detects elevated muscular sodium concentration in Duchenne muscular dystrophy," Neurology, vol. 77, no. 23, pp. 2017-2024, 2011, doi: 10.1212/wnl.0b013e31823b9c78.
[5] M. A. Weber et al., "Permanent muscular sodium overload and persistent muscle edema in Duchenne muscular dystrophy: a possible contributor of progressive muscle degeneration," Journal of Neurology, vol. 259, no. 11, pp. 2385-2392, 2012, doi: 10.1007/s00415-012-6512-8.
[6] P. A. Bottomley, "Human in vivo NMR spectroscopy in diagnostic medicine: Clinical tool or research probe?," Radiology, vol. 170, no. 1, pp. 1-15, 1989.
[7] S. E. Ogier et al., "A frequency translation system for multi-channel, multi-nuclear MR spectroscopy," IEEE Transactions on Biomedical Engineering, vol. 68, no. 1, pp. 109-118, 2020.
[8] T.W. Nixon, Y. Liu, H.M. DeFeyter, S. McIntyre, R.A. de Graaf. Hardware developed for phase and frequency locking of interleaved MRI and DMI studies, Proceedings of the 2021 ISMRM and SMRT Annual Meeting and Exhibition. Online, International Society of Magnetic Resonance in Medicine, May 2021.
Figures