4968
Magnetic Resonance Imaging to Identify Chronicity and Treatability of Migraine and Chronic Headache Disorders1Vanderbilt University Medical Center, Nashville, TN, United States, 2Philips Healthcare, Gainesville, FL, United States, 3Philips Healthcare, Nashville, TN, United States
Synopsis
Migraine Headaches (MH) are the 6th leading cause of years lived with disability worldwide, affect 15% of the US population, and are nearly twice as prevalent in veterans who have been deployed. Our study focuses on pathology of the greater occipital nerve (GON), which occurs with a high prevalence in individuals with a history of trauma (i.e. traffic accidents, explosions, falls). In our pilot MRI scans and operative experience, migraines associated with GON pathology displayed a pathologic thickening of the nerve validated intraoperatively. These results present MRI as a potential biomarker of headache pathologies of MH associated with GON pathology.
Introduction
Migraine Headaches (MH) are the 6th leading cause of years lived with disability (YLD) worldwide, affect 15% of the United States population, and are nearly twice as prevalent in veterans who have been deployed 1–3. While the broad military population has a similar prevalence of migraines as civilians, up to 92% of military personnel with mild traumatic brain injuries (TBI) develop persistent headaches 3,4. Our study focuses on pathology of the greater occipital nerve (GON), which occurs with a high prevalence in individuals with a history of trauma (i.e. traffic accidents, explosions, falls). The GON originates in the neck from nerve fibers of the dorsal rami of the second and third spinal nerves and courses into the subcutaneous tissue of the scalp near the nuchal lines where it provides sensation to the skin of the occipital scalp. In our pilot MRI scans and operative experience, migraines associated with greater occipital nerve pathology have coincided with an observed pathologic thickening of the nerve at any of the six points of possible compression previously characterized by nerve surgeons 5–7. Magnetic resonance neurography (MRN) of the GON requires a high signal-to-noise ratio (SNR), high contrast between nerve and fat and between vasculature and muscle.8.The goal of this work was to develop an MRN protocol that allows to : i) track the GON from its origin in the neck to its entry into the subcutaneous tissue of the scalp near the nuchal lines, ii) identify potential compression points responsible for the headache symptoms, iii) validate these measures against clinician evaluation of intraoperative findings and the post-surgical outcomeMethods
An MRI study was performed at 3T (Philips Ingenia, Philips Healthcare, Best, Netherlands) using 16-channel neurovacualr coil on three volunteer patients with clinically reported migraines associated with GON pathology and scheduled surgical decompression. Fluid-sensitive fat suppressed T2-weighted sequences are the foundation of MR neurography techniques as they allow for the examination of peripheral nerve health by T2 signal intensity, as well as morphological assessment (nerve course, caliber, and size) 9. We developed three sub-millimeter resoution 3D isotropic MR neurography sequences to sufficiently visualize the GON but also identify the potential compression points like arteries, veins and muscles. The three 3D MR neurography sequences (T2-FFE, Fast Inversion Recovery, and 3D mDIXON TSE) provide superior T2-weighting but utilize different mechanisms to suppress the surrounding soft-tissue structures to create images with high nerve conspicuity. The T2-FFE utilizes a tissue selective pulse to provide enhanced T2 contrast while reducing the signal from fat and muscle. Fast Inversion Recovery (3D NerveVIEW) employs a tissue specfic inversion recovery pulse to suppress the signal from fat and muscle to produce a T2-weighted MR neurography image. Unlike the T2-FFE and NerveVIEW, the mDIXON TSE sequence uses a reconstruction post-processing technique to allow for the separation of the signal associated with the nerves (water) compared to other soft-tissues (fat). These three sequences were overlapped with same orientation along the nerve using Connectome Workbench version 1.4.2. Nerves thickness are reported as mean ± standard error. The MRI images were compared visually with intraoperative findings noted by the surgeon.Results
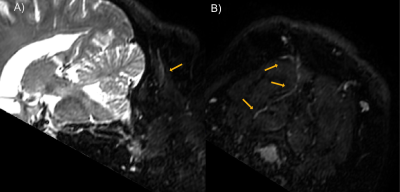
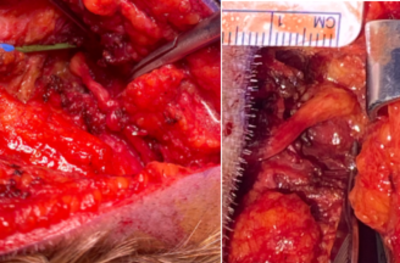
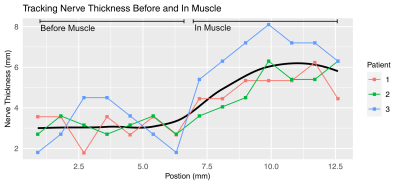
Maps of the occipital nerve’s course in a patient were achieved with a slightly oblique axial plane as shown in Figure 1. Pathological thickening can be noted at the location where nerve crosses the semispinalis capitis muscle. Surgical intraoperative findings shown in Figure 2 validated the MRI results, also identifying the most notable change in the nerve structure to be on the left side within the substance of the semispinalis capitis muscle. Figure 3 illustrates the quantification of nerve thickness from MRI images in three patients at points proximal to the semispinalis capitis muscle and after the nerve enters into the muscle. The first patient’s nerve had a mean thickness of 3.1 ± 0.2 mm before and 5.1 ± 0.2 mm within the semispinalis capitis muscle. The second and third patients also demonstrated increased nerve thickness, with changes from 3.1 ± 0.4 mm to 5.1 ± 0.4 mm and from 3.1 ± 0.5 mm to 6.8 ± 0.3 mm, respectively. Our results matched surgical observations showing an pathological increase in nerve thickness of the greater occipital nerve in all three patients suffering of migraines.Discussion and Conclusion
These results indicate that sub-millimeter resolution MRN and nerve tracking of the GON are capable of detecting pathologic changes in nerve structure. These changes were validated intraoperatively and may therefore be potential biomarkers of the headache pathologies of occipital neuralgia and MH associated with GON pathology. Furthermore, these findings suggest that nerve thickness assessments may be associated with the patient’s disease severity as well as the prognosis. Future work includes i) relating this finding to intraoperative quantitative findings, ii) compare results with healthy controls, and iii) investigate advanced diffusion models and quantitative magnetization transfer models that can account for edema, restricted diffusion in axons, and demyelinationAcknowledgements
We want to thank the Department of Plastic Surgery at Vanderbilt University Medical Center for funding this research and the Vanderbilt University Institute of Imaging at Vanderbilt University Medical Center for assistance and support with the MRI protocol development.References
1. Burch, R., Rizzoli, P. & Loder, E. The prevalence and impact of migraine and severe headache in the United States: Updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache 61, 60–68 (2021).
2. Vos, T. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015).
3. Bader, C. E., Giordano, N. A., McDonald, C. C., Meghani, S. H. & Polomano, R. C. Musculoskeletal Pain and Headache in the Active Duty Military Population: An Integrative Review. Worldviews evidence-based Nurs. 15, 264–271 (2018).
4. Dikmen, S., Machamer, J., Fann, J. R. & Temkin, N. R. Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 401–411 (2010).
5. Hwang, L., Dessouky, R., Xi, Y., Amirlak, B. & Chhabra, A. MR neurography of greater occipital nerve neuropathy: Initial experience in patients with migraine. Am. J. Neuroradiol. 38, 2203–2209 (2017).
6. Blake, P. & Burstein, R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J. Headache Pain 20, (2019).
7. Ascha, M., Kurlander, D. E., Sattar, A., Gatherwright, J. & Guyuron, B. In-Depth Review of Symptoms, Triggers, and Treatment of Occipital Migraine Headaches (Site IV). Plast. Reconstr. Surg. 139, 1333e-1342e (2017).
8. Van der Cruyssen, F. et al. Magnetic resonance neurography of the head and neck: state of the art, anatomy, pathology and future perspectives. Br. J. Radiol. 20200798 (2021). doi:10.1259/bjr.20200798
9. Chhabra, A. et al. MR Neurography: Past, Present, and Future. Am. J. Roentgenol. 197, 583–591 (2011).
Figures