4967
Diffusion Tensor Imaging Biomarkers for Chronic Pain Following Mild Traumatic Injury1Departments of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 2Department of Kinesiology, School of Health and Human Sciences, Indiana University Purdue University Indianapolis, Indianapolis, IN, United States, 3Department of Anesthesia, School of Medicine, Indiana University School of Medicine, Indianapolis, IN, United States, 4Stark Neuroscience Research Institute, Indiana University School of Medicine, Indianapolis, IN, United States, 5Research and Development Services, Richard L. Roudebush VA Medical Center, Indianapolis, IN, United States
Synopsis
To investigate the neuropathogenesis of post-traumatic headache following mild traumatic brain injuries (mTBI), this study applied diffusion tensor imaging and functional magnetic resonance imaging to explore relationships between brain structural and functional changes and sensitization, pain inhibitory capacity, psychological factors and headache pain in mTBI subjects. The results found structural and functional alterations in mTBI. Also, the results showed that DTI metric differences between mTBI subjects and controls can be used to predict pain/psychological measurements, which indicated the relationship between white matter disruption and measurements of endogenous pain modulation and psychological distress in mTBI.
Introduction
More than 1.7 million traumatic brain injuries (TBI) occur in adults each year in the United States, with mild TBIs (mTBI) accounting for the majority of these injuries1. Among symptoms of mTBI, post-traumatic headache (PTH) is one of the worst with prevalence rates ranging from 30-90%2, 3. Therefore, understanding the neuropathogenesis behind PTH is crucial. Previous research indicates that mTBI is characterized by dysfunctional endogenous pain modulation, as evidenced by decreased endogenous pain inhibitory capacity and increasing trigeminal sensitivity following the mTBI event4, 5. Deficient pain inhibitory capacity increases risk for the development of persistent PTH5. Additionally, alterations in the brain have been associated with mTBI and chronic pain6, 7. This study investigated brain structural and functional changes using diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) in mTBI subjects to detect relationships with sensitization, pain inhibitory capacity, psychological factors and headache pain. We hypothesized that pain/psychological measurements can be predicted by diffusivity changes and functional connectivity (FC) alterations.Methods
Participants: Twelve mTBI individuals recruited from a Level I trauma hospital and 10 TBI-free controls were enrolled in this study. The mTBI participants completed study sessions at 1-2 weeks (V1), 1-month (V2), and 6-months (V3) post injury. Controls completed two study sessions separated by 6 months.Pain and psychological assessments: All participants completed quantitative sensory tests (QST) measuring trigeminal sensitization (pressure pain thresholds and temporal summation of pain on the head) and endogenous pain inhibition (conditioned pain modulation). Subjects also completed validated questionnaires measuring headache pain, depression, anxiety, post-traumatic stress symptoms, and pain catastrophizing. Before each image session (see in next section), the Defense and Veterans Pain Rating Scale (DVRS) assessing current pain was administered.
Image Acquisition: The mTBI subjects completed brain scans at 1-month and 6 months visits. Controls also completed brain scans during each of their two study sessions. T1-weighted, diffusion-weighted and resting-state MRI scans were acquired on the Siemens MAGNETOM 3T Prisma with 32-channel head coil. The data analysis herein is restricted to brain scans completed at the 1-month visit only.
Diffusion MRI data analysis: The diffusion-weighted images were preprocessed followed the pipelines in previous publication in the FMRIB Software Library8, 9. DTI metrics including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (DR), and axial diffusivity (DA) were calculated and registered to Montreal Neurological Institute space with Advanced Neuroimaging Tools nonlinear registration10. Tract-based spatial statistics (TBSS) was applied to make whole-brain white matter skeleton. Skeletonized DTI metrics were averaged based on 17 headache-related region-of-interests (ROIs)11, 12. Generalized linear regression models were applied to:
1) calculate between group within-ROIs DTI metrics difference at the 1-month visit;
DTI metrics=β1*group+β2*age+β3*sex+β4*DVRS
2) predict pain/psychological measurements in mTBI subjects at 1-month and 6 months by using within-ROIs DTI metrics at 1-month;
Pain/psychological measurement=β1*(DTI metrics)+β2*age+β3*sex+β4*DVRS
3) compare predictive power between patients’ and controls’ DTI metrics at 1-month to pain/psychological measurements at 1-month and 6 months by adding an interaction term (group) in this model:
Pain/psychological measurement=β1*(DTI metrics)+β2*(DTI metrics)*group+β3*age+β4*sex+β5*DVRS
Resting-state fMRI data analysis: Resting-state images were preprocessed using CONN toolbox with typical preprocessing steps13. FC differences were calculated based on 40 independent components between patient and control with control of age, gender, and DVRS. The statistical threshold was set as p<.001 at the voxel-level, and as p<.05 at the false-discovery-rate (FDR)-corrected cluster-level. Cluster-level significance was tested using a nonparametric permutation testing with 5000 permutations. Cortical region with significant FC differences were then applied to predict the pain/psychological measurements.
Results
DTI results: The mTBI subjects exhibited significantly lower DA in the left cingulum cingulate gyrus and forceps major (p<0.05) compared to controls (Regression Model 1). The results (Table 1 and 2) also showed several significant correlations (p<0.05) between pain/psychological measurements and mTBI subject’s within-ROIs DTI metrics (Regression Model 2). The results (Table 3 and 4) revealed several significant (p<0.05) interaction effects in the 3rd prediction model, in which the correlation between the pain/psychological measurements and within-ROIs DTI metrics differed between mTBI subjects and controls.fMRI results: FC between one independent component and whole brain was found to be lower in mTBI subjects than control in precuneous cortex, cingulate gyrus, and posterior division regions (p<0.05). However, no significant correlations were evident between FC differences and pain/psychological measurements.
Discussion
This study interrogated DTI metrics and FC differences between mTBI subjects and controls, and using those image measurements to predict pain/psychological measurements. Within the mTBI sample at 1-month post injury, greater DA in left corticospinal tract, left anterior thalamic radiation, and near the insular cortex was associated with decreased pain inhibition on the CPM test. Also at 1-month, trigeminal sensitization was association with decreased FA at right cingulum hippocampus and increased DR of left anterior thalamic radiation. Finally, the relationship between the DTI metrics of the sagittal stratum, left superior longitudinal fasciculus, and the forceps major at 1-month and posttraumatic stress and depression at 6 months significantly differed between controls and mTBI subjects.Conclusion
The present study demonstrated brain structural and functional differences between mTBI subjects and controls at 1-month following injury, which also predicted pain/psychological measurements. Our findings suggest that measures of endogenous pain modulation and psychological distress might relate to white matter disruption following mTBI.Acknowledgements
The authors have no conflict of interest.References
1. Langlois JA, Rutland-Brown W and Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation 2006; 21: 375-378.
2. Lucas S, Hoffman JM, Bell KR, et al. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014; 34: 93-102.
3. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. Jama 2008; 300: 711-719.
4. Carey C, Saxe J, White FA, et al. An exploratory study of endogenous pain modulatory function in patients following mild traumatic brain injury. Pain medicine 2019; 20: 2198-2207.
5. Naugle KM, Carey C, Evans E, et al. The role of deficient pain modulatory systems in the development of persistent post-traumatic headaches following mild traumatic brain injury: an exploratory longitudinal study. The Journal of Headache and Pain 2020; 21: 1-12.
6. Lepage C, de Pierrefeu A, Koerte IK, et al. White matter abnormalities in mild traumatic brain injury with and without post-traumatic stress disorder: a subject-specific diffusion tensor imaging study. Brain imaging and behavior 2018; 12: 870-881.
7. Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. PAIN® 2013; 154: 2160-2168.
8. Smith S, Bannister PR, Beckmann C, et al. FSL: New tools for functional and structural brain image analysis. NeuroImage 2001; 13: 249.
9. Wu Y-C, Harezlak J, Elsaid NM, et al. Longitudinal white-matter abnormalities in sports-related concussion: A diffusion MRI study. Neurology 2020; 95: e781-e792.
10. Avants BB, Tustison N and Song G. Advanced normalization tools (ANTS). Insight j 2009; 2: 1-35.
11. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487-1505.
12. Chong CD, Peplinski J, Berisha V, et al. Differences in fibertract profiles between patients with migraine and those with persistent post-traumatic headache. Cephalalgia 2019; 39: 1121-1133.
13. Whitfield-Gabrieli S and Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity 2012; 2: 125-141.
Figures
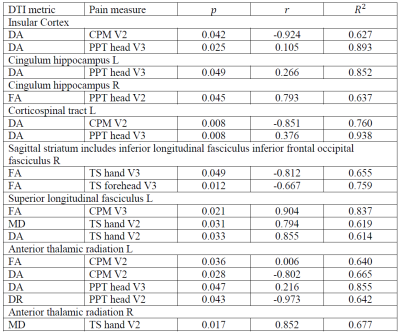
Table 1. Correlations between pain measurements and patients’ within-ROIs DTI metrics. TS: Temporal summation of pain: measures endogenous facilitation of pain. PPT: pressure pain threshold: Measures sensitization of the head area. CPM: Conditioned pain modulation: measures endogenous pain inhibition. V2: second visit. V3: third visit. r: correlation coefficient.
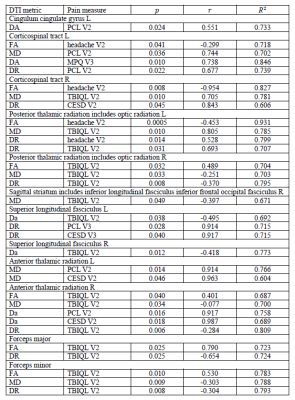
Table 2. Correlations between psychological measurements and patients’ within-ROIs DTI metrics. TBIQL: TBI Quality of Life Headache pain scale: measures subjective experience of headache symptoms for those with TBI. PCS: Pain Catastrophizing Scale. PCL: Posttraumatic stress symptoms questionnaire. CEDS: Depression scale. V2: second visit. V3: third visit. r: correlation coefficient.
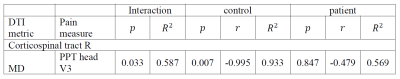
Table 3. Between-group correlation (correlation between pain measurements and within-ROIs DTI metrics) differences.
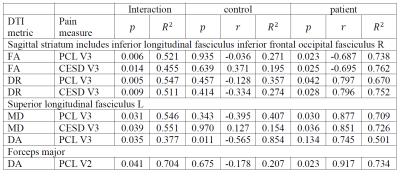
Table 4. Between-group correlation (correlation between psychological measurements and within-ROIs DTI metrics) differences.