4966
Predicting Stereotactic Radiosurgery Dose Maps from Pre-Therapy MR Images using a Deep Neural Network1Electrical Engineering, University of South Florida, Tampa, FL, United States, 2Department of Cancer Physiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States, 3Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States, 4Quantitative Imaging Shared Service, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States, 5Department of Oncologic Sciences, University of South Florida, Tampa, FL, United States
Synopsis
Stereotactic Radiosurgery (SRS) of asymptomatic brain metastases provides lasting tumor control with only minor side effects to healthy brain. An active research area is the development of models to predict tumor response to a given dose of Radiation Treatment (RT) from analysis of pre-RT and post-RT MR images (i.e., the forward problem). Here we propose an approach to train a deep neural net on pre-RT MR images of patients with Breast Cancer Metastases to the Brain (BCMB), for predicting RT dose maps that will yield desired/target tumor voxel intensities on post-RT MR images (i.e., the inverse problem).
Purpose
Treatment planning is a time-consuming and labor-intensive, but critical, process in radiotherapy of cancer. Some of the manual aspects of treatment planning can be alleviated with automated solutions that use rule-based, atlas-based and prior knowledge-based methods to plan the delivery of the prescribed radiation dose to targeted tumors while sparing organs-at-risk (OAR). However, such automated treatment planning solutions typically do not provide voxel-level predictions of RT dose that would be optimal for specific outcomes [1-6]. Voxel-level treatment planning would be beneficial for two tasks: one would be a reduction in labor-intensive steps to contour tumors and OARs, and the second would be for prescribing heterogeneous dose distributions to achieve optimal response within solid tumors that are spatially heterogeneous. Models to accomplish the first task require training data with ground truth pertaining to normal and pathologic anatomy, while models to accomplish the second task require ground truth on voxel-level tumor response to RT. Multiparametric MRI (mpMRI) images, particularly Apparent Diffusion Coefficient (ADC) of water maps, contain a wealth of information that is mechanistically relatable to voxel-level tumor response to therapies [7]. Here we have analyzed standard-of-care T1-weighted unenhanced (T1w) and contrast-enhanced (T1wCE), T2-weighted (T2w), Fluid-Attenuated Inversion Recovery (FLAIR) images and ADC maps acquired pre-RT and post-RT in patients with Breast Cancer Metastases to the Brain (BCMB) who received SRS. Our first aim is to identify post-RT vs. pre-RT changes on mpMRI that are correlated with response to SRS in BCMB. Our second aim is to use these putative response criteria as the voxel-level outcome measure for training a deep neural network on pre-RT T1w, T1wCE, T2w, FLAIR images and ADC maps to predict the optimal RT dose map for producing a prescribed/desired voxel-level change within the tumor volume on each image type.Method
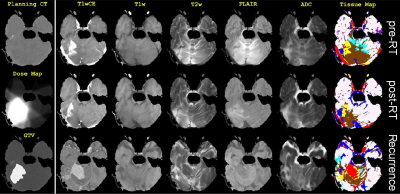
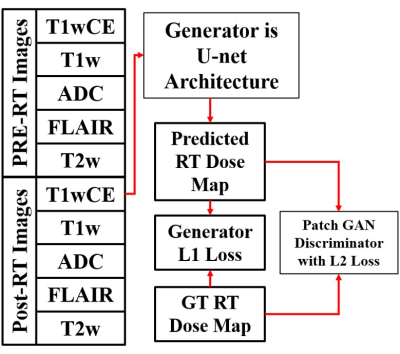
In this retrospective IRB-approved study, planning CT images and associated RT dose maps, and T1w, T1wCE, T2w, FLAIR images and ADC maps acquired pre-RT, post-RT (15-158 days), and at tumor recurrence (54-831 days) in twenty-five BCMB patients were curated from our Radiology and Radiation Oncology databases. These patients received a mean SRS dose of 21 Gy (range:15-30 Gy) in 1-5 fractions. mpMRI images from all scan dates were co-registered to the planning CT using MIRADA-RTx (Mirada Medical, Denver, CO, USA). Gross Tumor Volume (GTV) contours and the RT dose map associated with the planning CT could be applied to the mpMRI images after co-registration. Voxel intensities on T2w, FLAIR, T1w and T1wCE images were calibrated using two reference normal tissues [8]. Intensity-calibrated voxels on co-registered mpMRI images were assigned to objectively-defined tissue types [9]. Pre-RT vs. post-RT changes in voxels within the GTV on ADC and calibrated T2w, FLAIR, T1w and T1wCE images were analyzed for differences between responding and recurrent BCMB lesions. We modified the Multi-Modal Generative Adversarial Network (mm-GAN) [10], a variant of pix2pix framework, and trained it on intensity calibrated pre-RT and post-RT mpMRIs and the co-registered RT dose Map (Figure 2).Results and Discussion
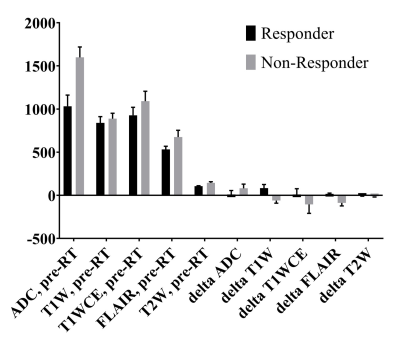
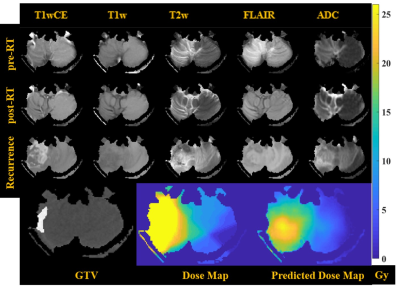
Co-registered image data from an example patient in this study are shown in Figure 1: the planning CT and associated RT dose map, and mpMRI scans acquired at three time points. Maps of objectively-defined tissue types shown in Figure 1 provide visual confirmation of the quality of the intensity-calibration process. Pixel intensities on calibrated images are comparable across patients and scan dates. The mean and SE values for pre-RT mpMRI and delta (post-RT minus pre-RT) changes across all analyzed responding and non-responding tumors are shown in Figure 3. The mean pre-RT ADC and T1wCE values within the GTV were higher in non-responding tumors compared with responding tumors. Interestingly, delta T1w within the GTV was positive in responding tumors but negative in non-responding lesions. Delta FLAIR within the GTV was also positive in responding tumors and negative in non-responding lesions. A similar effect was observed on delta T1wCE though the difference was not statistically significant. Unexpectedly, there were no significant differences on delta ADC between responding and recurrent lesions, possibly due to the long time interval (15-158 days) between RT and post-RT MRI. Based on these preliminary observations, the target “desirable” post-RT change within the GTV was chosen to be +119 on delta T1w and +22 on delta FLAIR, with no change relative to pre-RT on the other post-RT images within or outside the GTV in a proof-of-concept demonstration of the trained mmGAN network. The predicted and ground truth RT dose maps are compared and shown in Figure 4. The actually delivered dose map necessarily includes dose to all tissues along the beam paths, while this is not the case with the model predicted RT dose map.Conclusion
We present a preliminary demonstration of a framework for training an mmGAN model to predict SRS dose maps for voxelwise optimization of local control of BCMB tumors. Further experiments on additional data and cohorts are required to understand and validate this model.Acknowledgements
No acknowledgement found.References
[1] Lee H et al., "Fluence-map generation for prostate intensity-modulated radiotherapy planning using a deep-neural-network," Scientific Reports (2019) 9:15671.
[2] Nguyen D et al., "A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning," Scientific Reports (2019) 9:1076.
[3] Meerbothe T, "A physics guided neural network approach for dose prediction in automated radiation therapy treatment planning," Master of Science thesis at the Delft University of Technology, 2021.
[4] Ahn SH et al., "Deep learning method for prediction of patient-specific dose distribution in breast cancer,” Radiation Oncology (2021) 16:154.
[5] Ma M et al., "Dose distribution prediction in isodose feature‐preserving voxelization domain using deep convolutional neural network,” Medical Physics 46(7):2978-2987, 2019.
[6] Murakami Y et al., "Fully automated dose prediction using generative adversarial networks in prostate cancer patients," PLoS ONE (2020) 15(5):e0232697.
[7] Galbán CJ et al., “Diffusion MRI in early cancer therapeutic response assessment,” NMR Biomed (2017) 30:e3458.
[8] Stringfield O et al., "Multiparameter MRI predictors of long-term survival in glioblastoma multiforme," Tomography (2019) 5(1):135-144.
[9] Hawkins SH et al., “MRI Predictors of Response to Anti-PD1 Immune Checkpoint Inhibition, Bevacizumab and Hypofractionated Stereotactic Irradiation in Patients with Recurrent High Grade Gliomas,” Proc ISMRM, 1238, 2018.
[10] Sharma A and Hamarneh G, "Missing MRI pulse sequence synthesis using multi-modal generative adversarial network," IEEE Trans Med Imaging (2019) 39(4):1170-1183.
Figures