4960
Evidence of meningeal inflammation in multiple sclerosis: a combined in vivo 11C-PBR28 MR-PET and post-mortem study1Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden, 4Department of Neurology, 4. Karolinska University Hospital, Stockholm, Sweden, 5Neurology, Massachusetts General Hospital, Boston, MA, United States, 6Massachusetts General Hospital, Boston, MA, United States, 7Beth Israel Deaconess Medical Center, Boston, MA, United States, 8Department of Neurology, UMass Memorial Medical Center, Worcester, MA, United States, 9Systems and Biomedical Engineering Department, Faculty of Engineering, Cairo University, Giza, Egypt, 10Department of Neuroscience, University of California, San Diego, CA, United States, 11Neurology Section, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy, 12Division of Neuroscience, Department of Brain Sciences, Imperial College London, London, United Kingdom
Synopsis
In multiple sclerosis (MS), neuropathological studies suggest that meningeal inflammation involving T-, B-lymphocytes, plasma cells, macrophages may trigger underlying cortical neuroinflammation and demyelination. Using MR-PET targeting the18kDa mitochondrial translocator protein, which is overexpressed in activated glia/macrophages, we detected in 49 MS patients cortical inflammatory changes along with neuroinflammation in both meninges and juxtacortical white matter. Meningeal inflammation correlated with worse neurological disability and cognitive performance. Histochemistry performed in meningeal tissue from 40 post-mortem progressive MS cases confirmed in vivo findings. This study provides in vivo PET imaging evidence implicating meningeal neuroinflammation in the pathophysiology of MS.
Introduction
In multiple sclerosis (MS), neuropathological examinations suggest that cortical demyelination, which plays a key role in disability progression, may be, at least in part, related to inflammatory activity involving T and B lymphocytes, plasma cells, and macrophages in the overlying leptomeninges1. The in vivo study of meningeal inflammation in MS is hampered by the limitations of current imaging methods. Molecular imaging targeting of the 18kDa mitochondrial translocator protein (TSPO), which is overexpressed in activated glia and macrophages could represent a cell-specific approach to image neuroinflammation in vivo.Previous TSPO PET studies have provided in vivo evidence of increased TSPO expression in the MS cortex2,3,4. The exact nature of cortical glia/macrophage activation in MS patients remains controversial as it could simply reflect the spreading of pathology from underlying white matter (WM) or be also associated with meningeal inflammation as observed by histopathology. Additionally, the interpretation of TSPO cortical changes in MS could be biased by TSPO expression in endothelial cells.
Aims
In a heterogeneous cohort of 49 MS cases, we used MR-PET with 11C-PBR28, a second-generation TSPO radioligand, to investigate, relative to matched healthy controls (HC), i) 11C-PBR28 uptake in the cortex, underlying juxtacortical WM, meningeal compartment; ii) the role of endothelial TSPO binding in 11C-PBR28 cortical signal in MS iii) the relationship between meningeal 11C-PBR28 uptake in MS and clinical outcome. To validate in vivo findings, we performed histochemistry in meningeal tissue from 40 post-mortem progressive MS cases obtained from the UK MS tissue bank (Imperial College London).Methods
In vivo study. Twenty-one patients with secondary-progressive MS (SPMS), 28 with relapsing-remitting MS (RRMS) and 21 age- and TSPO affinity binding matched HC underwent 90-minutes 11C-PBR28 MR-PET (Siemens BrainPET). 11C-PBR28 MR-PET acquisitions from 15 additional subjects with amyotrophic lateral sclerosis (ALS) were also available for additional comparisons with MS data. Conventional anatomical 3T-MR scans were simultaneously acquired for cortical surface reconstruction, using FreeSurfer, and MR-PET image registration. Standardized uptake value (SUV) maps were created for 60-90-minute PET frame (1.25 mm isotropic voxels) and normalized (SUVR), to account for global differences across subjects, by a pseudo-reference region. Each individual SUVR map was projected to its surface and sampled at: i) mid cortical depth, ii) 4 mm above the cortex (meningeal/parameningeal tissue); iii) 4 mm below the cortex (WM). Prior to the surface-based group analyses, 11C-PBR28 surfaces were smoothed and registered into a common template. A general linear model in FreeSurfer assessed vertexwise differences across the different regions (mid cortical depth, parameningeal tissue and WM) in 11C-PBR28 SUVR between i) MS patients vs HC; ii) ALS vs HC. In meningeal areas showing abnormally increased 11C-PBR28 uptake in MS, the corresponding SUVR were extracted to investigate, using linear regression models, the relationship between meningeal TSPO expression and physical disability (Expanded Disability Status Scale, EDSS) and cognitive impairment (Symbol Digit Modalities Test, SDMT). Age and TSPO affinity binding were included as covariates of no interest.Endothelial binding was assessed using 2-tissue compartmental model with an irreversible, compartment that accounts for the endothelial TSPO binding5 in a subset of subjects who underwent PET scans with arterial blood sampling.Post-mortem study. Using immunohistochemistry, we assessed the number of cells expressing different monocyte/macrophage markers (MHC-II, CD68, CD14, CD11c, CD163) in the meningeal infiltrates of post-mortem progressive MS. For each examined MS case, 4 tissue blocks containing undamaged meninges were analyzed and the mean cell number was calculated.Results
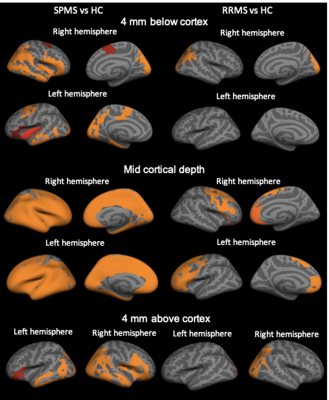
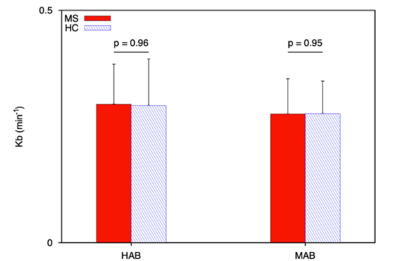
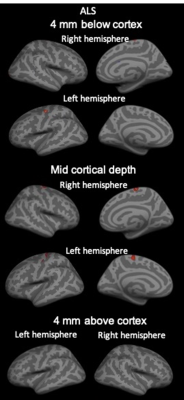
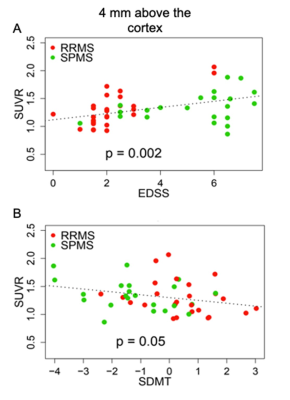
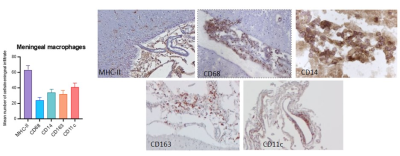
Our results showed diffuse cortical 11C-PBR28 uptake in MS patients (Figure 1). Endothelial TSPO binding did not represent a main factor accounting for 11C-PBR28 differences between MS vs HC (Figure 2). In contrast to ALS patients that showed increased 11C-PBR28 uptake in the motor cortex and juxtacortical WM but not in meningeal tissue (Figure 3), MS patients showed diffuse cortical inflammation accompanied by increased TSPO signal in both meningeal tissue and juxtacortical WM (Figure 1). These changes were diffuse in SPMS, while presented with a more focal pattern in RRMS. Increased TSPO levels in meningeal tissue were associated with increased EDSS and lower SDMT scores (p=0.002 and p=0.05, respectively) (Figure 4). We found that meningeal infiltrates in the subarachnoid spaces, particularly in B-cell follicle-positive MS cases, were characterized not only by accumulation of B lymphocytes, but also by a large proportion of activated monocyte/macrophage expressing MHC-class II. Then, differential expression of further markers, such as CD11c, marker of antigen presentation, as well as monocyte marker CD14, activated phagocytic macrophage marker CD68, and iron-receptor CD163 were found in the same infiltrates in smaller proportion (Figure 5).Discussion and Conclusions
Our findings in meningeal tissue could be reminiscent of meningeal inflammation with activation of macrophages/monocytes found in progressive MS cases, but also described by previous neuropathological reports on early MS6. Additionally, there is ample evidence that CNS borders are populated by myeloid cells from adjacent bone marrow niches, strategically placed to supply innate immune cells under homeostatic and pathological conditions7. This study provides in vivo PET imaging evidence implicating meningeal neuroinflammation in the pathophysiology of the disease.Acknowledgements
This study was supported partly by NIH 1 R21 NS126737-01, National MS Society (NMSS) RG 4729A2/1, RG-1882-30468 and partly by the Department of Defense (DoD) US Army W81XWH-13-1-0112 Award. Elena Herranz was supported by NMSS TA-1905-34039.References
1. Bogdan F. Gh. Popescu, Istvan Pirko, Claudia F. Lucchinetti. Pathology of Multiple Sclerosis: Where Do We Stand? Bogdan F. Gh. Popescu, Istvan Pirko, Claudia F. Lucchinetti. Continuum (Minneap Minn) 2013 Aug; 19(4 Multiple Sclerosis)
2. Herranz E, Giannì C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016; 80:776–790.
3. Singhal T, O’Connor K, Dubey S, et al. Gray matter microglial activation in relapsing vs progressive MS: A [F-18]PBR06-PET study. Neurol Neuroimmunol Neuroinflamm 2019; 6: e587.
4. Politis M, Giannetti P, Su P, et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 2012; 79:523–30
5. Rizzo G, Veronese M, Tonietto M, et al. Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data.. J Cereb Blood Flow Metab 2014; 34:1060–9
6. Bevan RJ, Evans R, Griffiths L, Watkins LM, Rees MI, Magliozzi R, Allen I, McDonnell G, Kee R, Naughton M, Fitzgerald DC, Reynolds R, Neal JW, Howell OW. Meningeal inflammation and cortical demyelination in acute multiple sclerosis Ann Neurol. 2018 Dec;84(6):829-842
7. Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, Baker W, Papadopoulos Z, Drieu A, Blackburn S, Kanamori M, Brioschi S, Herz J, Schuettpelz LG, Colonna M, Smirnov I, Kipnis J. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021 Jul 23;373(6553):eabf7844.
Figures