4959
Association of White Matter Integrity of Locus Coeruleus Pathways to Hypothalamus and Sleep Apnea in Military Mild Traumatic Brain Injury1National Intrepid Center of Excellence, Bethesda, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Walter Reed National Military Medical Center, Bethesda, MD, United States
Synopsis
Sleep disturbances are common following traumatic brain injury (TBI). The intent of this study is to characterize the role of the noradrenergic locus coeruleus (LC) for sleep maintenance in service members following mild TBI by associating microstructural features, derived from diffusion MRI, of the LC–noradrenergic (NA) system with objective sleep measures. We found that severity of sleep apnea, particularly in rapid eye movement stage during sleep, significantly correlated with microstructural changes in the LC pathways to hypothalamus in mTBI participants. This result suggests sleep apnea following mild TBI may be modulated by sympathetic activity via pathways interconnecting LC and hypothalamus.
Introduction
Sleep apnea after mild traumatic brain injury (mTBI) is common in service members (SMs). The locus coeruleus (LC)-noradrenergic (NA) system plays an important role in regulating arousal/wakefulness 1, sleep behaviors 2, and higher cognitive functions . Animal studies have suggested that chronic sleep disruption, a feature of sleep apnea, can activate inflammatory processes in wake-activated neurons, e.g. LC 3. Postmortem studies in patients with severe TBI have shown loss of LC neurons 4. The intent of this study is to characterize the role of the noradrenergic LC for sleep maintenance in SMs following mTBI by associating structural features of the LC–NA with objective sleep measures. The pathways from LC to hypothalamus are of interest in the study of primary sleep disorders as well as in sleep changes occurring after TBI. We hypothesize that mTBI SMs with sleep apnea have greater structural changes of the LC-NA system than those of mTBI SMs without sleep apnea; and disruption of projections to the LC can result in prevention of respiratory plasticity in chronic mTBI patients, leading to sleep apnea.Methods
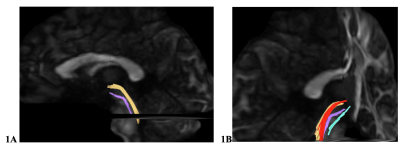
Diagnosis of mTBI during enrollment occurred via a multi-layered structured interview process screening for every TBI and potential concussive event across the entire lifetime, using a modification of the Ohio State University TBI Identification instrument 5. This instrument consists of an interview that systematically identifies all events during lifetime that may have resulted in a TBI. Each injury is classified during a consensus meeting as a TBI, potential concussive event, or no-injury. In this retrospective study, we included 158 male SMs with mTBI and 46 non-TBI controls. Participants underwent MRI scans including structural MRI, multi-shell (3 shells, b=3000, 2000, 1000 s/mm² and 90 diffusion directions for each shell) multi-band (acceleration factor=3) diffusion MRI (dMRI) on a 3T MRI scanner using a 32-channel, phased-array head coil. Brainstem fibers in the LC-NA system such as white matter (WM) tracts interconnecting the LC and the hypothalamus (LC-HT), the LC and the thalamus (LC-TH) were the target WM pathways (Fig. 1). LC-HT and LC-TH streamlines were reconstructed using generalized q-ball imaging (GQI) 6 with deterministic tractography by placing seed regions of interest in the LC, and target ROIs in the HT and TH, respectively. Bundle-specific metrics, e.g. quantitative anisotropy (QA) and generalized fractional anisotropy (GFA), were quantified. Sleep data (TBI group only) included subjective sleep measure, Pittsburgh Sleep Quality Index, and objective polysomnography (PSG) data; these data along with the post-traumatic stress disorder CheckList – Civilian Version (PCL-C) were used for correlation analysis. PSG test was administered during their overnight sleep. The sleep variables included: sleep stage architecture, including rapid eye movement (REM) stage sleep, and non-REM stage 3 sleep, i.e., slow-wave sleep, sleep efficiency, Daytime Sleepiness Scores, apnea-hypopnea index (AHI, the number of apneas or hypopneas recorded per hour of sleep). Sleep apnea diagnosis was determined using a cut-off AHI score at AHI ≥ 5 7. While controlling age covariate, mixed modeling was used to evaluate group difference of diffusion metrics; and partial Pearson correlation was used for correlation analysis of regional diffusion metrics and PSG data. Multiple comparisons were corrected using the Bonferroni method and statistical significances were set at adjusted p < 0.05. An absolute value of correlation coefficient larger than 0.2 was considered significant.Results
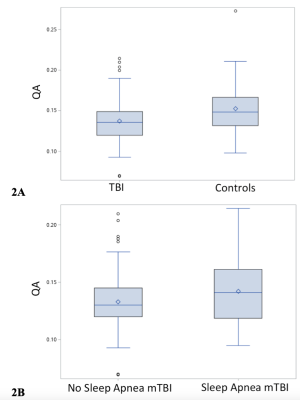
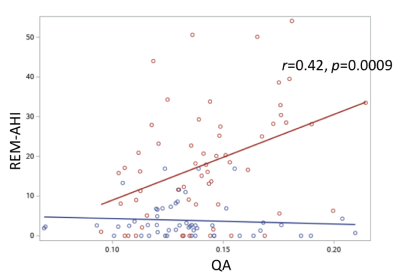
Fig. 1 shows the example of reconstructed LC-HT and LC-TH pathways. mTBI participants as a group were older than controls (40.4 ± 5.2 vs 36.5 ± 8.1 years old). For group comparisons of diffusion metrics of the LC-HT and the LC-TH pathways, mTBI had significantly lower QA in the right LC-HT (Fig. 2A) and in the bilateral LC-TH pathways. Sleep apnea mTBI had higher QA than no sleep apnea mTBI in the right LC-HT (Fig. 2B) and in the left LC-TH, and higher GFA in the left HC-TH than those of non-sleep apnea mTBI. The number of AHI in REM stage (REM-AHI) (y-axis in Fig. 3) significantly correlated with QA in the right LC-HT pathways (x-axis) in mTBI participants (p=0.0009, r= 0.42 for sleep apnea mTBI, p=0.0007, r= 0.32 for all mTBI participants) (Fig. 3). Total PCLC score correlated inversely with QA in the left LC-HT pathways in mTBI participants (p=0.029, r= -0.21). There was no significant correlation between diffusion metrics in the LC-TH pathways and sleep measures.Discussion / Conclusions
Both LC-HT and LC-TH tracts are anatomically consistent with the path of central tegmental tract which branches into the thalamus and hypothalamus, respectively. The results of this study suggest that diffusion metrics derived from multi-shell dMRI can reflect the severity of brainstem white matter disruption following brain injury. Furthermore, we have shown that microstructural alternation in the LC-HT bundles, but not in the LC-TH bundles, was associated with REM-AHI in mTBI patients. We will evaluate blood pressure contributing to sleep apnea because of association between REM sleep and sympathetic activity 8. Relatively higher diffusion anisotropy of the HC-HT bundles in mTBI participants with sleep apnea than in those without sleep apnea may be due to peri-lesional gray matter glial hypertrophy and reactive gliosis 9,10,11. These findings suggest that neuroinflammation of LC-NA system plays an important role in the pathogenesis of WM microstructural changes.Acknowledgements
Disclaimer
The views expressed in this abstract are those of the authors and do not necessarily reflect the official policy of the Department of Defense or the U.S. Government.
References
1. O’Donnell, J., Ding, F. & Nedergaard, M. Distinct Functional States of Astrocytes During Sleep and Wakefulness: Is Norepinephrine the Master Regulator? Curr. Sleep Med. Reports 1, 1–8 (2015).
2. Samuels, E. & Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr. Neuropharmacol. 6, 235–253 (2008).
3. Poe, G. R. et al. Locus coeruleus: a new look at the blue spot. Nature Reviews Neuroscience vol. 21 644–659 (2020).
4. Valko, P. O. et al. Damage to Arousal-Promoting Brainstem Neurons with Traumatic Brain Injury. Sleep 39, 1249–1252 (2016).
5. Walker, W. C. et al. The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: Description of study and characteristics of early participants. Brain Inj. 30, 1469–1480 (2016).
6. Fang-Cheng Yeh, Wedeen, V. J. & Tseng, W.-Y. I. Generalized q-Sampling Imaging. IEEE Trans. Med. Imaging 29, 1626–1635 (2010).
7. Young, T. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA 291, 2013–2016 (2004).
8. Trinder, J. et al. Autonomic activity during human sleep as a function of time and sleep stage. J. Sleep Res. 10, (2001).
9. Budde, M. D., Janes, L., Gold, E., Turtzo, L. C. & Frank, J. A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134, 2248–2260 (2011).
10. Hutchinson, E. B., Schwerin, S. C., Avram, A. V., Juliano, S. L. & Pierpaoli, C. Diffusion MRI and the detection of alterations following traumatic brain injury. J. Neurosci. Res. 96, 612–625 (2018).
11. Bouix, S. et al. Increased Gray Matter Diffusion Anisotropy in Patients with Persistent Post-Concussive Symptoms following Mild Traumatic Brain Injury. PLoS One 8, e66205 (2013).
Figures