4949
Trabecular bone imaging using 3D ultrashort echo time (UTE) Cones at the fat peak frequency: feasibility study1Department of Radiology, University of California, San Diego, San Diego, CA, United States, 2General Electric Healthcare, San Diego, CA, United States, 3Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
High susceptibility levels at the marrow/bone interface may significantly reduce T2* of marrow, leading to trabecular bone volume overestimation when imaged using conventional MRI sequences. The presence of fat in bone marrow further complicates trabecular bone imaging due to chemical shift artifacts. In this study, an ultrashort echo time MRI (UTE-MRI) technique focused on the fat peak frequency was investigated to image trabecular bone ex vivo and in vivo. This technique was shown to improve trabecular bone imaging by minimizing chemical shift artifacts as well as susceptibility related short T2* effects, thereby providing more accurate estimation of trabecular bone structure.
INTRODUCTION
Bone has an extremely short apparent transverse relaxation time (T2*) and is typically rendered “invisible” when imaged using conventional pulse sequences with echo times (TEs) of a few milliseconds (1). High resolution MRI (e.g., voxel size~0.2mm) can indirectly visualize trabecular bone as dark regions surrounded by marrow. With data post-processing, it is possible to obtain the 3D architecture of trabecular bone (2). Such visualization has been used to track bone structure changes in response to medical treatments (3–5) as well as for finite element analysis-driven mechanical competence assessment (6). High susceptibility at the marrow/bone interface together with multiple fat peaks may significantly reduce marrow T2*, resulting in trabecular bone volume overestimation. Ultrashort echo time MRI (UTE-MRI) allows acquiring signals from tissues with short T2* such as bone and its neighboring marrow, which could potentially avoid bone volume overestimation. However, UTE-MRI is sensitive to chemical shift artifacts, which may lead to strong spatial blurring of bone structures. Alternatively, MR imaging at the fat peak frequency is hypothesized to minimize marrow-related chemical shift artifacts. Bone is expected to be off-resonance in fat-centered imaging. However, bone signal, while detectable with UTE-MRI, is much lower than marrow signal due to its low proton density and short T2*, leading to negligible water-associated off-resonance artifact. This study aimed to investigate the feasibility of using UTE-MRI at the fat peak frequency for more accurate depiction of the trabecular bone structure in the human calcaneus ex vivo and in vivo at 3T.METHODS
Ex vivo study: Whole ankle specimens and sectioned distal tibial samples (n=11) were scanned at room temperature on a 3T clinical scanner (MR750, GE Healthcare Technologies, WI). The following four 3D MR sequences were performed: 1) 3D gradient echo (GRE) sequence (TR=20, TE=4.4 ms) at the water peak frequency; 2) 3D-UTE-Cones sequence (TR=7.6, TE=0.032 ms) at the water peak frequency; 3) 3D-UTE-Cones sequence (TR=7.6, TE=0.032 ms) at the fat peak frequency; and 4) 3D-UTE-Cones at the fat peak frequency with five different TEs (TE=0.032, 1.1, 2.2, 3.3, 4.4 ms). Typical imaging parameters included: Field-of-view (FOV)=8 cm, acquisition matrix=384×384, slice thickness=0.6mm, voxel size=0.2×0.2×0.6mm3, slices=84-160, scan time=5-10 minutes, respectively.In vivo study: The ankle of a 43-year-old man was scanned using the setup described above, with only minor differences to shorten the scan time by limiting the number of slices to 84 with a slice thickness of 1mm. Zero-interpolation was used during image reconstruction, resulting in a reconstructed nominal voxel size of 0.2×0.2×0.5 mm3 under 5.5 minutes scan time.
Results
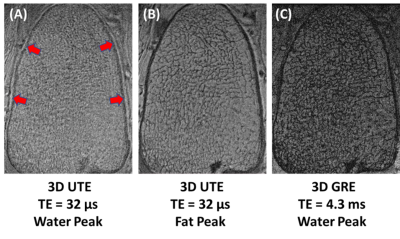
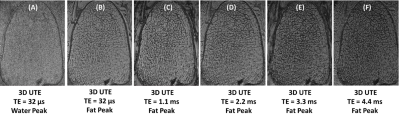
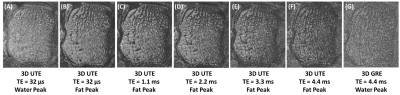
Figure 1 shows high-resolution imaging of a sectioned distal tibia sample. Strong chemical shift artifacts were observed in 3D-UTE-Cones imaging at the water peak, leading to obscured trabecular structures within the sample. In contrast, 3D-UTE-Cones imaging at the fat peak shows high signal for marrow fat, with excellent depiction of trabecular bone structures. Longer TEs led to more signal loss in marrow fat, likely due to the susceptibility-related T2* shortening. 3D-GRE sequence showed excellent depiction of marrow fat, but with more signal loss compared with the UTE-Cones images at the water peak. Figure 2 shows 3D-UTE-Cones and GRE imaging of a whole ankle specimen from a 65-year-old male donor. UTE-Cones at the water peak shows strong chemical shift artifacts (red arrows), which are greatly reduced in UTE-Cones imaging at the fat peak. The clinical GRE sequence showed excellent contrast, but with obvious trabecular bone overestimation. Figure 3 shows 3D-UTE-Cones imaging of the same whole ankle specimen at different TEs, again with more marrow signal loss at longer TEs. Figure 4 demonstrates in vivo calcaneal trabecular bone images of a 43-year-old male volunteer. Trabeculae were seen with higher contrast in images acquired at the fat peak frequency and greater marrow signal loss was observed in images acquired at longer TEs.DISCUSSION
The feasibility of the UTE-MRI sequence at the fat peak frequency in trabecular bone imaging was examined ex vivo and in vivo. The 3D-UTE-Cones sequence at the fat peak frequency improved the contrast in visualizing the trabeculae while minimizing the fat-to-water chemical shift artifact. Potential chemical shift artifact from bone (off-resonance in fat-centered imaging) was not observed due to the low proton density of water in bone. Bone volume was apparently higher in images performed at higher TEs, likely due to the marrow’s shortened T2* caused by the susceptibility phenomenon at the marrow/bone interface. More accurate depiction of trabecular bone structure also allows more reliable finite element analysis of its mechanical properties. Nevertheless, the accuracy of the visualized bone volume needs to be investigated in future studies comparing MRI and microcomputed tomography data.CONCLUSION
The proposed UTE-Cones sequence at the fat peak frequency was shown to improve trabecular bone imaging by enhancing the contrast between bone and marrow, in addition to avoiding the potential bone overestimation caused by chemical shift artifacts and the high level of susceptibility at the marrow-bone interface.Acknowledgements
The authors acknowledge grant support from the NIH (R01AR068987, R01AR062581, and R01AR075825), Veterans Affairs (I01RX002604 and I01CX001388), and GE Healthcare.References
1. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013;26:489–506 doi: 10.1002/nbm.2906.
2. Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19:731–764 doi: 10.1002/nbm.
3. Zhang XH, Liu XS, Vasilic B, et al. In vivo μMRI-based finite element and morphological analyses of tibial trabecular bone in eugonadal and hypogonadal men before and after testosterone treatment. J. Bone Miner. Res. 2008;23:1426–1434 doi: 10.1359/jbmr.080405.
4. Wehrli FW, Rajapakse CS, Magland JF, Snyder PJ. Mechanical implications of estrogen supplementation in early postmenopausal women. J. Bone Miner. Res. 2010;25:1406–1414 doi: 10.1002/jbmr.33.
5. Rajapakse CS, Leonard MB, Bhagat YA, Sun W, Magland JF, Wehrli FW. Micro-MR imaging-based computational biomechanics demonstrates reduction in cortical and trabecular bone strength after renal transplantation. Radiology 2012;262:912–920 doi: 10.1148/radiol.11111044.
6. Rajapakse CS, Kobe EA, Batzdorf AS, Hast MW, Wehrli FW. Accuracy of MRI-based finite element assessment of distal tibia compared to mechanical testing. Bone 2018;108:71–78 doi: 10.1016/j.bone.2017.12.023.
7. Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: A preliminary study at 3.0 Tesla. J. Magn. Reson. Imaging 2007;26:787–793 doi: 10.1002/jmri.21072.
8. Du J, Chiang AJT, Chung CB, et al. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn. Reson. Imaging 2010;28:178–184 doi: 10.1016/j.mri.2009.06.002.
9. Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J. Magn. Reson. 2010;207:304–311 doi: 10.1016/j.jmr.2010.09.013
10. Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T 2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn. Reson. Med. 2017;00:1–9 doi: 10.1002/mrm.26908.
11. Ma Y, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed. 2016;29:1546–1552 doi: 10.1002/nbm.3609.
12. Ma Y, Lu X, Carl M, et al. Accurate T 1 mapping of short T 2 tissues using a three-dimensional ultrashort echo time cones actual flip angle imaging-variable repetition time (3D UTE-Cones AFI-VTR) method. Magn. Reson. Med. 2018;80:598–608 doi: 10.1002/mrm.27066.
Figures