4939
Time-Dependent Diffusion Tensor Imaging and Modeling of Aging Muscles: Correlations to Muscle Strain, Histology and Physical Assessment1Radiology, UC San Diego, San Diego, CA, United States, 2Physics, San Diego State University, San Diego, CA, United States, 3Physiology and Membrane Biology, UC Davis, Davis, CA, United States
Synopsis
Diffusion modeling (RPBM) of the time dependence of the skeletal muscle diffusion eigenvalues was applied to probe tissue changes with age. λ2 (TM=30 ms) was significantly lower in seniors; permeability and the residence time from RPBM were significantly positively correlated with grip size and 200 m walk. Significant negative correlations of λ1 and λ2 to age and to physical λ2 / λ3 to collagen matrix angle and to systolic/diastolic blood pressure were seen. λ2 and λ3 were significantly positively correlated to the projection of the strain on the diffusion eigenvector corresponding to the secondary eigenvalue.
Introduction
Skeletal muscle time-dependent diffusion tensor imaging allows the computation of indices that reflect underlying muscle tissue microarchitecture. We measure the time dependence of skeletal muscle diffusion eigenvalues (λ1, λ2, λ3) using a STEAM-EPI DTI sequence to extract tissue microstructure in young and senior subjects using the Random Permeable Barrier Model. Time-dependent diffusion eigenvalues and model parameters were correlated to histological analysis of medial gastrocnemius (MG), physical assessment and to 3D strain / strain rate indices from dynamic MRI on the same cohort.Methods
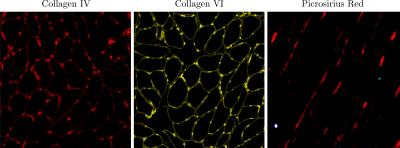
All imaging studies were performed after IRB approval on a GE 3.0 Tesla scanner on seven young (31 ± 8 years) and six older (75 ± 5 years) participants. The DTI protocol used a custom-built STEAM-EPI DTI sequence. Six non-collinear gradient directions with a nominal b-factor of 400 s/mm2 were used to map the direction-dependent diffusion at ten values of the mixing time, TM (20 ms to 600 ms). Diffusion data were pre-processed for eddy current and susceptibility induced artifacts, and denoised prior to computing the diffusion eigenvalues. The corrected full b matrix that accounted for the diffusion and imaging gradients was calculated as outlined in [1]. For the nominal b=0 images, the b-value varied from 2.3 s/mm2 at TM= 20 ms to 72 s/mm2 at TM= 700 ms and for the nominal b=400 s/mm2 images, the b-value varied from 371 s/mm2 at TM=20 ms to 465 s/mm2 at TM= 700ms. The RPBM model [2] is characterized by three parameters: the free diffusion coefficient, D0, the membrane permeability, κ, and the membrane surface to volume ratio, S/V, the myofiber size, a. The RPBM fits were made to the time dependence of the average of λ2 and λ3 ($$$ D\perp $$$); the values of $$$ D\perp $$$ were the average over the medial gastrocnemius. Dynamic imaging was performed using a custom-built compressed-sensing-based velocity encoded phase contrast imaging; 3D strain, strain rate, and fiber aligned strains and strain rates were extracted in the MG [3]. Physical assessment for muscle strength was made using the following metrics: Hand Grip (left and right), Chair stand time, # of chair stands completed, the timing for 200 m and for 400 m walk respectively, systolic (SBP) and diastolic (DBP) pre- and post-assessment blood pressure. Biopsies were performed from the MG muscle under ultrasound guidance; cross-sectional analysis was performed to quantitate collagens I, III, IV, V, and VI content, whereas longitudinal sections were stained with picrosirius red to determine matrix orientation, phi. Since collagen IV surrounds each cell, these images were also used to determine muscle fiber cross-sectional area (fCSA) and the Ferret diameter, (dF) (Figure 1).Results
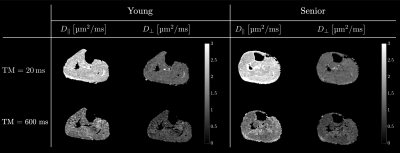
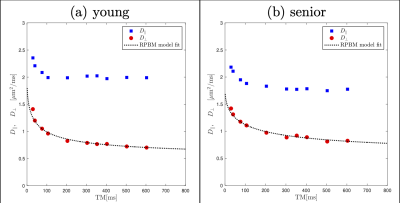
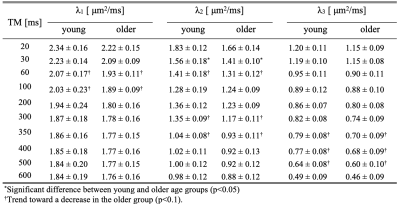
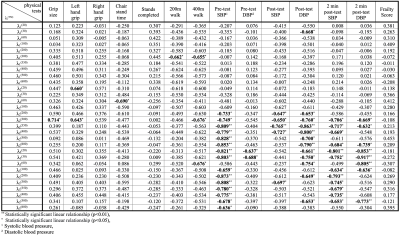
Figure 2 shows the diffusion eigenvalues for a young and senior subject at two TMs. Figure 3 is the fit of the RPBM model to the average of λ2 and λ3. λ2 (TM=30 ms) was significantly lower in the older cohort while λ1, λ2, λ3 showed a trend to decrease with age (Table 1). Of the modeling derived parameters, residence time showed a trend to increase with age. Significant negative correlations of λ1 and λ2 to age, chair stand time, 200 m and 400 m walk, and significant positive correlation to left-hand grip were seen (Table 2). Significant negative correlations of λ2 and λ3 to phi and to pre-test systolic and to post-test diastolic blood pressure were seen. Permeability and the residence time from RPBM were significantly positively correlated with grip size and with 200 m walk, respectively. Diffusion eigenvalues and strain indices showed that the projection of the strain on the diffusion eigenvector corresponding to the secondary eigenvalue was significantly positively correlated to λ2 (TM=300 to 600ms) and λ3 (TM=20, 30, 200-500 ms).Discussion
Age-related decrease in the eigenvalues including the lead eigenvalue and/or mean diffusivity has been attributed to the atrophy of the muscle fiber [4]. While muscle fiber atrophy can potentially result in smaller secondary and tertiary eigenvalues the muscle fiber length is much longer than the mean diffusion distance even at the longest diffusion times used in the current study. In one model of diffusion in the muscle, λ2 arises from a diffusive pathway within the endomysium; it would be more likely to occur in one direction leading to a λ2 that is sensitive to the cross-sectional orientation of the fibers. Based on this reasoning and the fact that the lack of cross-sectional symmetry increases with age [5], it was predicted that the maximum change with age would be seen in λ2 [3,4,6]. This is experimentally observed in the current study. λ2 also shows a significant negative correlation to the collagen matrix alignment to the muscle fiber (phi) at three mixing times (30, 60, and 100 ms). Phi increases with age and the age-related differences in the alignment of the collagen matrix may impact diffusion in the endomysium. The positive significant correlation of λ2 and λ3 to the strain index along the secondary diffusion eigenvector may indicate that larger strains (muscle cross-sectional radial expansion) may result in larger fiber cross-sections leading to larger diffusion along those directions.Conclusions
Time-dependent diffusion eigenvalues and modeling provide insight into tissue microstructural changes.Acknowledgements
This work was supported by the National Institute on Aging Grant No. R01AG056999.References
[1] Mattiello J et al., Magnetic Resonance in Medicine, 1997;37:292–300
[2] Fieremans E et al., NMR in biomedicine, 30(3), 10.1002/nbm.3612.
[3] Malis V et al., Front Physiol., 2020 Dec 3;11:600590
[4] Galbán CJ et al., The journals of gerontology, 2007;62:453–458
[5] Andersen JL et al., Scand J Medicine Sci Sports, 2003;13:40–47
[6] Galbán CJ et al., Eur J Appl Physiol., 2004;93:253–262
Figures