4930
MRI-derived contractility index as an early measure of thoracic radiation therapy-induced subclinical cardiotoxicity1Medical College of Wisconsin, Milwaukee, WI, United States, 2Washington University, St Louis, MO, United States
Synopsis
Radiation therapy (RT) plays an integral role in treating a number of thoracic cancers, despite risks of radiation-induced cardiotoxicity. The purpose of this study is to investigate the capability of advanced the MRI-generated contractility index (ContractiX) for early detection of RT-induced cardiotoxicity in a pre-clinical rat model of thoracic cancer RT. While EF increased post-RT, peak systolic strain and ContractiX significantly decreased post-RT, with more relative reduction in ContractiX compared to strain. Therefore, ContractiX is a sensitive early marker for detection of subclinical cardiac dysfunction post-RT in pre-clinical models.
Introduction
With increased rates of lung cancer survival, cardiovascular toxicity has become a major cause of mortality in lung cancer patients. Along with systemic therapies, radiation therapy (RT) plays an integral role in treating advanced lung cancer, despite the high incidence of RT-induced cardiovascular diseases. The current paradigm for cardiac function assessment and management of cardiovascular disease relies primarily upon the assessment of ejection fraction (EF). However, EF alone is limited in both its diagnostic and prognostic ability, as ventricular remodeling could compensate for regional cardiac dysfunction to maintain normal EF. MRI tagging1 allows for quantitative assessment of myocardial contractility on a regional basis. As regional changes in myocardial contractility frequently occur before cardiac damage2,3, this makes regional cardiac dysfunction potentially reversible and myocardial strain-derived parameters early indicators of cardiotoxicity. In this study, we present the contractility index (ContractiX) as a sensitive marker for early detection of subclinical cardiac dysfunction post-RT in a rat model of lung cancer, with end goal to help with risk stratification and treatment management to avoid cardiovascular complications.Methods
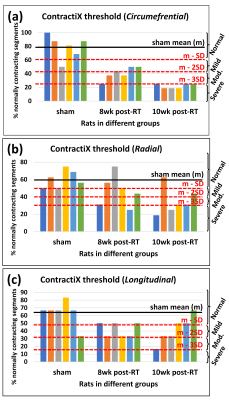
A total of 18 salt-sensitive (SS) adult female rats, aged ~10 weeks, were randomized into two groups: sham-treated (N=6) and cardiac RT (N=12). The RT rats received whole-heart radiation to 24 Gy using 3 equally-weighted fields with an X-RAD SmART Irradiator. The rats were imaged on a small-animal 9.4T Bruker MRI scanner using a 4-element surface coil at 8- and 10-weeks post-RT and the results compared to sham irradiated rats. The MRI scan included both cine and tagged images. The cine images were analyzed using the cvi42 software to measure EF. The tagged images were analyzed using the SinMod4 technique to measure myocardial circumferential, radial, and longitudinal peak-systolic strains (Ecc, Err, Ell) in different LV segments based on the American Heart Association (AHA) 17-segment model. For clarity of presentation, strain measurements are presented in absolute value. The ContractiX parameter is introduced as a new measure of heart contractility performance, derived from regional strain measurements in different segments in the LV AHA segmental model. ContractiX is generated as the percentage of normally contracting myocardium for each rat, where a myocardial segment was considered normally contracting if its peak systolic strain exceeded a threshold value, calculated as the mean minus one standard deviation (SD) of the strain values from all sham rats. A ContractiX threshold value for differentiating between sham and RT rats was defined based on measurements from the sham rats, as further explained in the Results section. ContractiX values were calculated from different strain components (circumferential (ContractiX-circ), longitudinal (ContractiX-long), and radial (ContractiX-rad)) and changes in ContractiX post-RT were compared to those in EF and strain values. ANOVA analysis was conducted for multiple comparisons between different rat groups.Results
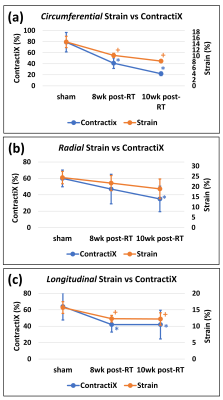
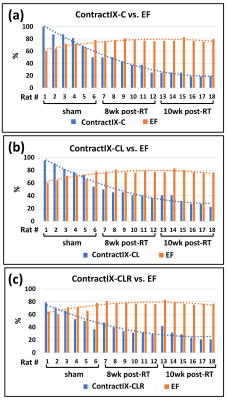
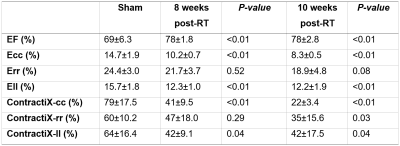
LVEF significantly increased post-RT compared to the sham rats, as shown in Table-1. Despite increased LVEF, different strain measurements (Ecc, Err, Ell) decreased post-RT, as shown in Table-1. The ContractiX parameters (ContractiX-circ, ContractiX-rad, ContractiX-long) significantly reduced post-RT (Table-1 and Figure-1), where the degree of reduction was most in the circumferential direction, followed by that in the longitudinal direction, and then in the radial direction. Nevertheless, the degree of reduction in ContractiX was always larger than the reduction in strain in all directions, i.e. the ContractiX parameter was more sensitive than myocardial strain to RT. Figure-2 shows ContractiX calculated from different combinations of strain components for all rats, along with the corresponding EF values. While normal EF was maintained post-RT, all ContractiX parameters showed continuous decrease post-RT. This can be explained by the nonlinear inverse relationship between EF and ContractiX in which ContractiX spans a wide range of measurements between 19% and 100% for rats in different groups despite normal LVEF (> 60%) for all rats. Figure-3 shows % normally contacting segments for the rats in different groups (sham, 8 weeks post-RT, 10 weeks post-RT) in the circumferential, radial, and longitudinal directions, where the (mean - n SD) thresholds can distinguish between different degrees of cardiac dysfunction in different rat groups, especially in the circumferential and radial directions.Discussion and Conclusions
EF increased in this rat model post-RT, as previously shown5, attributed to undergoing ventricular remodeling and hypertrophy to maintain cardiac output in the face of RT injury. The results demonstrate the sensitivity of ContractiX as an early marker of subclinical cardiac dysfunction post-RT despite normal global function. While normal EF was maintained post-RT, ContractiX showed continuous decrease from the sham to 8 weeks post-RT and then to 10 weeks post-RT rats. In conclusion, the newly defined ContractiX parameter showed to be an early marker of subclinical cardiac dysfunction post-RT, with higher sensitivity than other measures of cardiac function, such as EF and strain.Acknowledgements
Study funded by Daniel M. Soref Charitable TrustReferences
1. Ibrahim el SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques--pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36.
2. Kongbundansuk S, Hundley WG. Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovasc Imaging. 2014;7:824-838.
3. Ibrahim el S, Baruah D, Croisille P, Stojanovska J, Rubenstein J, Frei A, Schlaak R, Lin C, Pipke JL, Lemke A, Xu Z, Klaas A, Brehler M, Flister MJ, Laviolette P, Gore E, Bergom C. Cardiac Magnetic Resonance for Early Detection of Radiation Therapy-Induced Cardiotoxicity in a Small Animal Model. JACC Cardio-Oncology. 2021;3:113-130.
4. Arts T, Prinzen FW, Delhaas T, Milles JR, Rossi AC, Clarysse P. Mapping displacement and deformation of the heart with local sine-wave modeling. IEEE Trans Med Imaging. 2010;29:1114-1123.
5. Schlaak RA, Frei A, Schottstaedt AM, Tsaih SW, Fish BL, Harmann L, Liu Q, Gasperetti T, Medhora M, North PE, Strande JL, Sun Y, Rui H, Flister MJ, Bergom C. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am J Physiol Heart Circ Physiol. 2019;316:H1267-H1280.
Figures