4926
ECG-free 2D cardiac cine MRI with data-driven clustering1Physics and Biology in Medicine Graduate Program, University of California, Los Angeles, Los Angeles, CA, United States, 2Department of Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Division of Cardiology, David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System, Los Angeles, CA, United States, 4Department of Radiation Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 5Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Conventional 2D cardiac cine imaging relies on ECG-gating whereas self-gated approaches mitigate ECG-dependency by assuming that cardiac motion is periodic with well-defined frequencies. This assumption breaks down when patients have irregular cardiac rhythm. We propose a segmented Cartesian golden step balanced steady-state free precession sequence (bSSFP) with motion navigators and a clustering algorithm to alleviate the dependency on regular motion assumptions and to emphasize the intrinsic similarity of mechanical motion. Compared to standard ECG-gated 2D bSSFP cine performed in normal sinus rhythm, initial validation using our approach achieved similar image quality and quantitative metrics for cardiac function.
Introduction
2D cardiac cine imaging1 is a widely used technique to evaluate cardiac structure and function. To resolve cardiac motion, ECG-gating is commonly applied, but self-gating is an alternative approach when the ECG is not useable. Traditional self-gating methods2-4 however, assume that the cardiac motion signal has a defined and narrow frequency range and resolve cardiac motion by filtering these signals5. For patients with highly irregular or chaotic cardiac rhythm, conventional gating methods have not been reliable and are inefficient in the clinical setting. We propose a segmented k-space, Cartesian golden-step balanced steady-state free precession sequence (bSSFP) with motion navigators and a clustering algorithm that alleviates the dependency on regular cardiac motion assumptions.Methods
A free-running Cartesian golden-step balanced steady-state free precession (bSSFP) sequence6 with segmented k-space acquisition was designed to (a) ensure adequate k-space coverage after data binning using our cluster algorithm, and (b) mitigate eddy current artifacts (Figure 1a). In this way, advantages of the golden step were preserved, and potential eddy current artifacts were mitigated. Extra k-space center lines were added every n TRs to act as motion navigators for the purpose of obtaining cardiac motion information (Figure 1b). With n=4, the corresponding temporal resolution of the navigators was approximately 20 ms.After data acquisition, a k-means7 clustering algorithm was performed on the motion navigator data for binning of the acquired data into cardiac phases prior to image reconstruction. First, motion navigator data were converted to 1D projection using IFFT. The spatial gradient of the projections was computed based on the projections and the gradient data from multiple coils were concatenated to form a data matrix X. Principal component analysis (PCA) of the data matrix identified the 20 largest principal components, which were used as inputs into the cluster algorithm. To mitigate the effects of random initial guesses, the k-means based algorithm was repeated until a stable solution was achieved. The cluster assignments which minimized the sum of in-cluster distances among all the clusters were used for data binning. Data from each cluster was assumed to represent one cardiac phase. Finally, the ESPIRiT8 reconstruction method was applied to every cluster bin to generate cine images. A summary of the workflow is illustrated in Figure 2.
To test our strategy, four patients with sinus rhythm (heart rate range 60 to 75 beats/min) were scanned on a 3.0T scanner (Skyra, Siemens) using our free-running segmented golden step Cartesian bSSFP sequence. Acquisition parameters were: flip angle (FA)=67 deg, slice thickness=8mm, matrix size=256*277, spatial resolution=1.4mm x 1.4mm x 8mm, TE/TR = 2.3ms /4.48ms, number of segments=20. Both images were acquired in horizontal and vertical long-axis views. Although our sequence was not ECG-triggered, the ECG signal was recorded as a reference. The scans were acquired under breath-holding (~18-20 secs) to minimize the impact of respiratory motion. To assess the performance of our cluster algorithm on distinguishing data with different motion states, we generated reference cine images with the same raw data using data binning based on the recorded ECG. The left ventricular ejection fraction (LVEF) and volumes were computed and the quantitative metrics for each method were compared using Bland-Altman analysis.
Results and Discussion
Figure 3 shows the systolic and diastolic images in two patients using our cluster-based cine imaging technique and traditional ECG-gating method. Qualitatively, there were no significant image artifacts. Left ventricular regional wall motion was normal in all visualized myocardial segments. The SNR and CNR for the systolic and diastolic phases of the cluster-based and ECG-gating method were 8.8±3.5(cluster-based) vs 7.5±2.7 (ECG-gating) and 6.2±2.0 (cluster-based) vs 6.1±1.9 (ECG-gating). The cluster-based method performed slightly better than ECG-gating on SNR/CNR, likely because our cluster-based method generally bins more data into the diastolic and systolic phases than other phases. Unlike the standard ECG-gating method which evenly distributes data into phase bins, the cluster-based method enhances the SNR/CNR but may do so at the expense of the other cardiac phases. This is acceptable in the setting of arrhythmias because the EF is a desired metric that is used as a determinant for many therapeutic options. The coefficients of variation were: 5.5% (LVEF), 9.0% (end-diastolic volume), 6.8% (end-systolic volume). The bias between the two methods was 0.02 (limits of agreement: upper 0.09, lower -0.05) for LVEF. Although our technique did not use ECG gating, typical filtering, or correlation strategies that have been used in previous self-gating methods, we were able to generate images of comparable quality as ECG-gating. These findings suggest early feasibility for using our image acquisition approach and clustering algorithm to distinguish data from different cardiac phases without assuming periodic cardiac motion or motion frequency range.Conclusion
Using a data-driven clustering approach to distinguish cardiac motion states is feasible. Compared to conventional ECG-gated cine, our image acquisition strategy and clustering approach achieved similar image quality in subjects with normal sinus rhythm, where both good qualitative and quantitative agreement were observed. Because no assumptions about rhythm regularity are required, the results should generalize to subjects with arbitrary cardiac rhythms. Confirmation of this preliminary conclusion in a larger set of patients with sinus rhythm and arrhythmias will be needed.Acknowledgements
No acknowledgement found.References
1. Atkinson, Dennis J., and R. R. Edelman. "Cineangiography of the heart in a single breath hold with a segmented turboFLASH sequence." Radiology 178.2 (1991): 357-360.
2. Larson, Andrew C., et al. "Self‐gated cardiac cine MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 51.1 (2004): 93-102.
3. Yerly, Jérôme, et al. "Coronary endothelial function assessment using self‐gated cardiac cine MRI and k‐t sparse SENSE." Magnetic resonance in medicine 76.5 (2016): 1443-1454.
4. Ingle, R. Reeve, et al. "Self‐gated fat‐suppressed cardiac cine MRI." Magnetic resonance in medicine 73.5 (2015): 1764-1774.
5. Heerfordt, John, et al. "Similarity‐driven multi‐dimensional binning algorithm (SIMBA) for free‐running motion‐suppressed whole‐heart MRA." Magnetic Resonance in Medicine 86.1 (2021): 213-229.
6. Scheffler, Klaus, and Stefan Lehnhardt. "Principles and applications of balanced SSFP techniques." European radiology 13.11 (2003): 2409-2418.
7. Likas, Aristidis, Nikos Vlassis, and Jakob J. Verbeek. "The global k-means clustering algorithm." Pattern recognition 36.2 (2003): 451-461.
8. Uecker, Martin, et al. "ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA." Magnetic resonance in medicine 71.3 (2014): 990-1001.
Figures
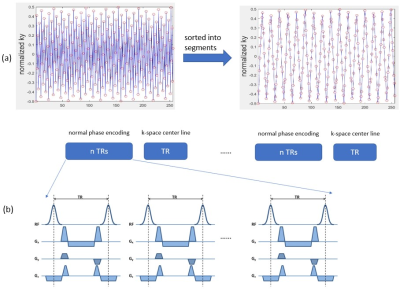
Figure 1. Original golden-step pulse sequence modifications. Our modifications reordered the original golden-step phase encoding to decrease the gap between adjacent phase-encoding lines. (a) Reordering of phase encoding lines into segments. The x-axis is the index for phase encoding lines and the y-axis represents locations of the lines in the ky dimension. The left panel plots the original golden-step trajectory and the right panel plots our modified segmented golden-step sequence trajectory. (b) Additional k-space center line navigators were added to track cardiac motion.
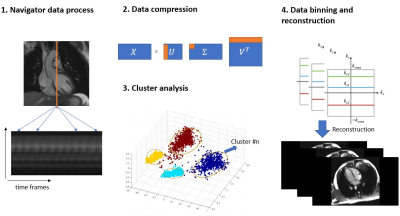
Figure 2. Workflow for cluster-based cine imaging: 1) Navigator data are converted and combined to a motion data matrix X. 2) Principal component analysis of X reduces high dimension data from N×T to compressed data M×T (where N>M). 3) Compressed data X’ are regarded as T data samples and used as inputs into the cluster algorithm. Outputs are T cluster numbers showing which samples were considered in one specific cluster. 4) Data binning and ESPIRiT reconstruction are performed according to cluster outputs and sequence trajectory.
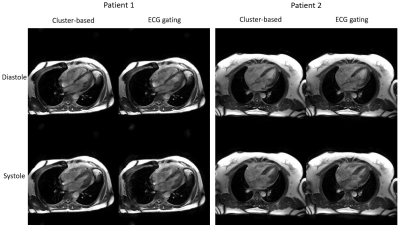
Figure 3. Illustrative comparison between images generated using our cluster-based technique and reference images using ECG-gating in two subjects. Good agreement in image quality and quantitative measurements were achieved between our cluster-based and reference images for both patients.