4921
Confirming conduction defect in fatty acid oxidation compromised heart using MRI strain imaging1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Healthy heart is a metabolically dynamic organ requiring large energy resource, and fatty acid oxidation (FAO) pathway predominantly supports this energy demands. Disorders of FAO pathways could range from mild and manageable to severe and life-threatening. In this study, we use MRI to evaluate the heart function in mitochondrial trifunctional protein (MTP) mutant rats, where clinical symptoms of this disorder overlap with other less severe FAO deficiencies and are often misdiagnosed. The results showed that MRI strain imaging revealed subclinical cardiac dysfunction in the MTP mutant rats with the degree of strain reduction depending on strain direction and regional location.
Introduction
Healthy heart is a metabolically dynamic organ requiring large energy resource, and fatty acid oxidation (FAO) pathway predominantly supports this energy demands. Normal healthy heart can switch between high energy fatty acids to low energy producing glycolysis and in fact it can utilize all energy sources depending on the health status, availability and need. Thus, heart is a versatile organ and can be metabolically flexible. Any disruption to this capability would result in dysfunction and ultimately organ failure. Gene mutations, physiological perturbations and stress would interrupt this metabolic flexibility resulting in impaired signaling, energy insufficiency, contractile dysfunction, and heart failure. Impairment of contractility could be due to either developmental deficiencies, structural damage or biochemical / metabolic deficiencies and therefore the genetic makeup is a significant contributor to the severity of heart failure. Disorders of FAO pathways could range from mild and manageable to severe and life-threatening. In many cases, the subclinical cardiac dysfunction is overlooked in this disorder even after an echocardiograph evaluation because cardiac dysfunction may be devoid of structural damage, along with only subtle cardiac dysfunction. However, if left untreated, this condition could progress into major cardiac complications and heart failure. In this study, we use MRI to evaluate the heart function in mitochondrial trifunctional protein (MTP) mutant rats, where clinical symptoms overlap with other less severe FAO deficiencies which complicate diagnosis, management and treatment options.Methods
Seven rats (3 MTP alpha variant heterozygous mutant Sprague-Dawley rats and 4 wild type controls) were imaged using an optimized MRI exam when they were 7 weeks of age. The MRI exam included both acquiring cine and tagging images covering the heart. The cine sequence imaging parameters were: repetition time (TR) = 7 ms, echo time (TE) = 2.1 ms, flip angle = 15°, matrix = 176x176, field of view (FOV) = 40x40 mm2, slice thickness = 1 mm, acquisition bandwidth = 526 Hz/pixel, # averages = 2, # cardiac phases = 20, scan time ~2 minutes/slice. The tagging sequence imaging parameters were similar to cine imaging, except for: matrix = 256x256, acquisition bandwidth = 375 Hz/pixel, # averages = 3, scan time = 4-5 minutes/slice. The cine images were analyzed using the cvi42 software package to measure ejection fraction (EF), while the tagged images were analyzed using the SinMod-based InTag software package to measure myocardial circumferential, radial, and longitudinal strain components. The results are represented as mean±SEM. Statistical analysis was conducted to determine significance of the measurement differences between the mutant and control rats (P<0.05 considered significant).Results
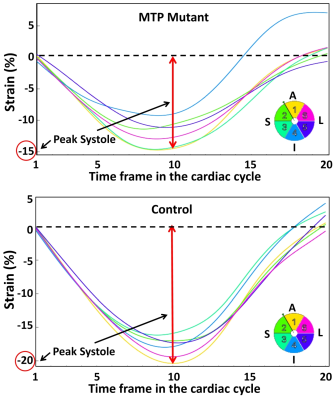
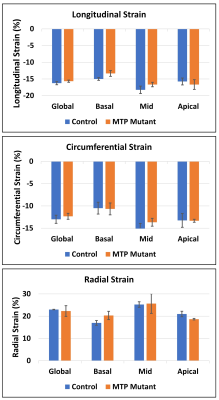
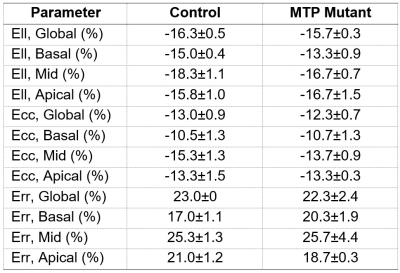
The MRI results revealed 10% reduction in EF (60.5±2.3% in control vs. 54.3±1.5% in MTP mutant rats). MRI also revealed reduced myocardial strain in the MTP mutant rats, compared to the control rats, in all strain directions (Figures 1-2), although the reductions in the circumferential and longitudinal strains were highly conspicuous than that in radial strain. Global longitudinal, circumferential, and radial strains in the MTP mutant rats were -15.7±0.3%, -12.3±0.7%, and 22.3±2.4%, respectively, vs. corresponding values of -16.3±5%, -13.0±0.9, and 23.0±0% in the control rats. The differences in strain measurements between the two groups were not statistically significant. Regional wise, most reductions in strain were in the basal and mid-ventricular segments compared to apical segments (Table 1). Basal longitudinal, circumferential, and radial strains in the MTP mutant rats were -13.3±0.9-10.7±1.3%, and 20.3±1.9%, respectively, vs. corresponding values of -15.0±0.4%, -10.5±1.3%, and 17.0±1.1% in the control rats. Mid-ventricular longitudinal, circumferential, and radial strains in the MTP mutant rats were -16.7±0.7%, -13.7±0.9%, and 20.3±1.9%, respectively, vs. corresponding values of -18.3±1.1%, -15.3±1.3, and 25.3±1.3% in the control rats. Apical longitudinal, circumferential, and radial strains in the MTP mutant rats were -16.7±1.5-13.3±0.3%, and 18.7±0.3%, respectively, vs. corresponding values of -15.8±1.0%, -13.3±1.5, and 21.0±1.2% in the control rats.Discussion and Conclusions
MRI is a valuable tool for detecting subtle changes in cardiac function in the MTP mutant rats. Especially, MRI strain imaging revealed subclinical cardiac dysfunction in the MTP mutant rats with the degree of strain reduction depending on strain direction and regional location. Therefore, a comprehensive MRI functional exam would be valuable for early detection of MTP mutation-induced cardiac dysfunction and potential development into heart failure.Acknowledgements
Study funded by Daniel Soref Trust.References
1. JL Merritt et al. Rev Endocr Metab Disord 2020; 21:479-493.
2. D Marsden et al. Genetics in Medicine 2021; 23:816-829.
3. EH Ibrahim et al. Magn Reson Imaging 2020; 73:130-137.
Figures