4920
Preservation of Human Hearts for Transplantation: Comparison of Metabolic Indicators after Conventional and Temperature Controlled Storage1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Department of Cardiovascular and Thoracic Surgery, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Center for Human Nutrition, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 6Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 7Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Preservation of human hearts for transplantation is important to optimize outcomes. Standard ischemic cooling methods are intended to preserve high energy phosphates but risk tissue damage due to freezing; an alternative with precise temperature control has emerged as an option. The efficacy of this technique in preserving high energy phosphates and other metabolites has not been examined. We compared conventional cold storage to a commercial device with precise temperature control for preservation of human hearts not suitable for transplant. There were no significant differences in high energy phosphates or other metabolites using precise temperature control compared to conventional cold storage.
INTRODUCTION
For end-stage heart failure, cardiac transplantation is the most effective therapy and donor heart preservation has a major influence on the transplant procedure's success. The conventional cold storage heart preservation strategy achieves near 0°C and is intended to reduce metabolism to preserve energy stores but is linked to extensive temperature fluctuations and may result in freeze damage1,2. One potential approach for expanding the donor pool is to strictly maintain the organ at a higher temperature between 4-8°C to prevent hypothermic injury1. This strategy may increase metabolism and the effect on high energy phosphates and other cardiac metabolites in human hearts during long ischemia periods has not been studied. Therefore, we attempted to compare three approaches: conventional cold preservation, preservation with precise temperature control, and preservation with precise temperature control and coronary perfusion. We hypothesized that strict temperature control at milder hypothermia would not lead to the depletion of high energy phosphates and other cardiac metabolism related metabolites in human donor hearts for transplantation.METHODS
All procedures were approved by the local organ procurement organization. Human donor hearts (n=10) that were not accepted for transplantation were assigned to one of three preservation techniques for 6 hours: 1) conventional cold storage (limited temperature control), 2) storage with precise temperature control of 4 to 8 °C (SherpaPak), or 3) precise temperature control plus coronary perfusion maintained at 4 to 8 °C (SherpaPak). However, due to technical issues with coronary perfusion in group 3, we are only reporting the data from static cold storage and precise temperature control. To acquire metabolic data, tissues from the left atrium, right ventricle, and left ventricle were obtained by biopsy after 6 hours of preservation with each technique.Phosphorus-31 NMR spectroscopy
Cardiac tissues were immediately snap-frozen and stored at -80 °C. For metabolic extraction, the frozen tissues were pulverized in liquid nitrogen and mixed with 4% perchloric acid. The perchloric extract of cardiac tissue was subsequently neutralized and reconstituted in D2O containing 1 mM EDTA and 0.5 mM 2,2-dimethyl-2-silapentane-5-sulfonate (DSS). 1H and proton-decoupled 31P-NMR spectra of heart tissue extracts were collected using a 14.1 T Varian spectrometer. The tissue concentration of metabolites was determined by deconvoluting 31P NMR resonances from 31P and 1H NMR spectra were analyzed. Metabolite ratios (e.g. PCr/Pi, ATP/Pi and PCr/ATP) were calculated as described previously2,3.
Liquid Chromatography / Mass Spectrometry
The frozen cardiac tissue was homogenized in 0.4 M perchloric acid containing 0.5 mM EGTA and kept on ice for 10 minutes before being centrifuged at 14,000 g at 4 °C for 10 minutes. All of the studies were performed using a Shimadzu LC-20AD liquid chromatography (LC) system with an API 3200 electrospray-ionization triple-quadrupole mass spectrometer (AB SCIEX, Framingham, MA). Metabolite ratios and other cardiac metabolism related metabolites were calculated as described previously4.
Data Analysis
The data were reported as the mean±SEM for both 31P-NMR (n=3 per group) and LCMS (n=4 per group), and statistical significance was determined using Welch's t-test (p ≤ 0.05).
RESULTS AND DISCUSSION
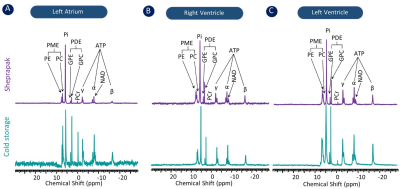
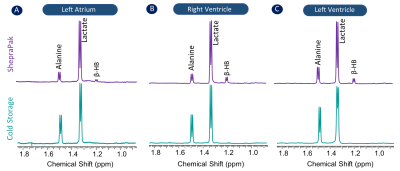
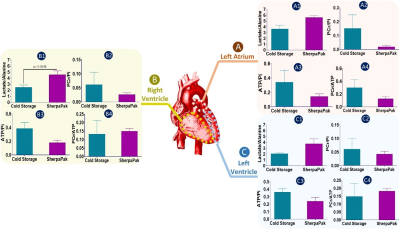
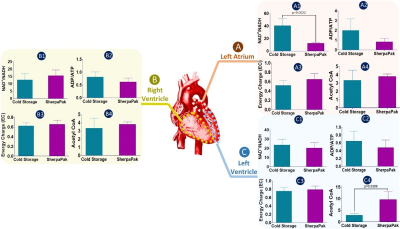
To compare the metabolic characteristics of the human heart preserved with temperature control, 31P, 1H NMR spectra and LC-MS data were analyzed. 31P spectra (Figure 1) showed resonances from adenosine triphosphate (ATP), nicotinamide adenine dinucleotide phosphate (NADP), inorganic phosphate (Pi), phosphocreatine (PCr), phosphomonoesters (PME) and phosphodiesters (PME) in tissues collected from the left atrium (Fig. 1A), right ventricle (Fig.1B) and left ventricle (Fig.1C) from hearts preserved by static cold storage and SherpaPak®. The 1H spectra indicate the resonance of lactate and alanine in tissues collected from the left atrium (Fig. 2A), right ventricle (Fig.2B) and left ventricle (Fig.2C) by group. Interestingly, the resonances from β-hydroxybutyrate can only be found in 1H spectra from tissues preserved using SherpaPak®, and this served as a notion to further analyze tissues using LCMS for other metabolites associated with cardiac metabolism. The metabolite ratios (lactate/alanine, PCr/Pi, ATP/Pi, and PCr/ATP) were calculated and shown in the (Fig 3. A1-A4) left atrium, (Fig 3. B1-B4) right ventricle, and (Fig 3. C1-C4) left ventricle. The lactate/alanine ratio was significantly higher in tissues from the right ventricle in the SherpaPak® (Fig. 3. B1) compared to static cold storage suggestive of higher anaerobic metabolism. The lactate/alanine ratios were not different between groups in tissue from the left ventricle (Fig. 3. C1) and left atrium (Fig. 3. A1). The energy metabolite ratios (PCr/Pi, ATP/Pi, and PCr/ATP) were similar in tissues collected from the left atrium (Fig. 3. A2-A4), right ventricle (Fig. 2. B2-B4), and left ventricle (Fig. 3. C2-C4). Furthermore, consistent with our 31P spectroscopy results, there were no significant changes observed in metabolite ratios (NAD+/NADH, ATP/ADP), energy charge, and Acetyl CoA by between groups, except for the NAD+/NADH ratio in the left atrium and Acetyl CoA in the right ventricle. These findings show that there are few differences in metabolic profiles of hearts preserved using precise temperature control versus conventional cold storage.CONCLUSION
Our studies suggest that heart storage at milder hypothermia with precise temperature control does not lead to depletion of high energy phosphates or other cardiac metabolites compared to conventional near 0°C cold storage. Phosphorus-31 NMR spectroscopy along with LCMS can be used to analyze different heart preservation methods.Acknowledgements
The devices
for this study were provided by Paragonix Technologies, Inc, Cambridge, MA and
the preservation solution by Bridge to Life Ltd, Northbrook, IL (to M.P.). This
work was supported by grants from the American Heart Association
(18POST34050049 to G.S.), the Robert A. Welch Foundation I-1804 (SCB) and NIH (P41-EB015908 to C.R.M.).
References
1. Naito N, Funamoto M, Pierson RN, D'Alessandro DA. First clinical use of a novel hypothermic storage system for a long-distance donor heart procurement. J Thorac Cardiovasc Surg. 2020 Feb;159(2):e121-e123. doi: 10.1016/j.jtcvs.2019.05.085. Epub 2019 Jul 10. PMID: 31420150.
2. Cobert ML, Merritt ME, West LM, Ayers C, Jessen ME, Peltz M. Metabolic characteristics of human hearts preserved for 12 hours by static storage, antegrade perfusion, or retrograde coronary sinus perfusion. J Thorac Cardiovasc Surg. 2014 Nov;148(5):2310-2315.e1. doi: 10.1016/j.jtcvs.2014.02.023. Epub 2014 Feb 8. PMID: 24642559; PMCID: PMC4126897.
3. Sharma G, Wen X, Maptue NR, Hever T, Malloy CR, Sherry AD, and Khemtong C. Co-Polarized [1-13C]pyruvate and [1,3-13C2]acetoacetate Provide a Simultaneous View of Cytosolic and Mitochondrial Redox in a Single Experiment. ACS Sensors, 2021.
4. Fu X, Deja S, Kucejova B, Duarte JAG, McDonald JG, Burgess SC. Targeted Determination of Tissue Energy Status by LC-MS/MS. Anal Chem. 2019 May 7;91(9):5881-5887. doi: 10.1021/acs.analchem.9b00217. Epub 2019 Apr 12. PMID: 30938977; PMCID: PMC6506803.
Figures