4919
Effect of Knockout of Two Acetyl-CoA Carboxylase Isoforms in the Isolated Mouse Heart on Oxidation of Exogenous and Endogenous Energy Sources1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Department of Chemistry, University of Texas at Dallas, Richardson, TX, United States, 6Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Florida, Gainesville, FL, United States, 7Department of Biochemistry and Molecular Biology, University of Florida, Gainesville, FL, United States
Synopsis
The product of acetyl-CoA carboxylase (ACC), malonyl-CoA, inhibits oxidation of long chain fatty acids by mitochondria. Cardiac-specific deletion of ACC-2 is associated with increased oxidation of fatty acids, as expected, the effects on glucose oxidation are controversial, and increased oxidation of stored triglycerides has been postulated. Expression of the other isoform, ACC-1, is preserved in ACC-2 mutant hearts, so alternative sources of malonyl-CoA may be important. We found that knock out of both isoforms was associated with a small increase in fatty acid oxidation, a small decrease in glucose oxidation, and little effect on oxidation of stored energy supplies.
INTRODUCTION
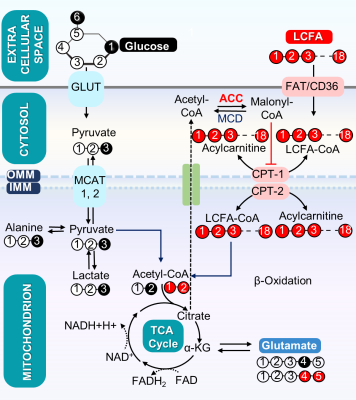
The major source of energy production in the heart is oxidation of long chain fatty acids (LCFA). Acetyl-CoA carboxylase (ACC) plays an important role in modulating fatty acid oxidation in the heart because it catalyzes carboxylation of acetyl-CoA, yielding malonyl-CoA, an inhibitor of fatty acid oxidation1. The dominant isoform in the heart is ACC-2 and knockout is associated with increased fatty acid oxidation and inhibition of glucose oxidation1. Others report an increase in glucose oxidation and suggest that oxidation of stored substrates are increased in ACC-2 mutant hearts2. Since ACC-1 persists in ACC-2 mutant hearts2, thus providing an alternative source of malonyl-CoA, we hypothesized that knockout of both acetyl CoA carboxylase isoforms (ACC-1 and ACC-2) would result in severe inhibition of glucose oxidation and a marked increase in fatty acid oxidation.METHODS
All procedures were approved by the Institutional Animal Care and Use Committee. In isolated Langendorff-perfused mouse hearts from 7-8 week old wild-type (WT), and Acetyl-CoA carboxylase 1 and 2 knockout mice (ACC KO), 13C-NMR isotopomer analysis was performed to examine substrate competition between glucose and fatty acid. Mouse hearts were removed shortly after cervical dislocation, cannulated through the aorta, and connected to a perfusion column device kept at 37°C using a temperature-controlled bath. Hearts were perfused retrogradely for 30 min at 100 cm H2O pressure with a modified Krebs-Henseleit (KH) buffer containing 8 mM [1,6-13C2] glucose and 0.4 mM [U-13C] long-chain fatty acids (LCFA) mixed with 0.75 percent bovine serum albumin (BSA). A thin-film oxygenator with a 95:5 O2:CO2 mixture was used to oxygenate the nonrecirculating buffer. A fluid-filled catheter in the left ventricle was placed to monitor heart rate throughout the perfusion. Coronary flow samples were taken into a gas-tight syringe at 5 and 25 min and examined using a blood gas analyzer to determine oxygen consumption (Instrumentation Laboratory, Lexington, MA). After 30 min of perfusion, hearts were snap-frozen, liquid nitrogen-pulverized, and extracted using 4% perchloric acid. The perchloric extract of cardiac tissue was subsequently neutralized and reconstituted in D2O containing 1 mM EDTA and 0.5 mM 2,2-dimethyl-2-silapentane-5-sulfonate (DSS). 1H and proton-decoupled 13C-NMR spectra of heart tissue extracts were collected using a 14.1 T spectrometer equipped with a 5-mm cryoprobe (Bruker Corporation, USA). The relative oxidation of [1,6-13C2]glucose, [U-13C]FA, and unlabeled endogenous substrates was determined by deconvoluting 13C NMR multiplets from glutamate using ACD/Spec Manager (ACD Labs, Canada) and multiplet ratios (e.g., triglycerides and glycogen). For isotopomer analysis, multiplet ratios were entered into tcaCALC (v. 2019). The data was reported as the mean SEM (n=4 per group) and statistical significance was determined using the Welch's t-test ("*": P≤ 0.05 and "**": P ≤0.01).RESULTS and DISCUSSION
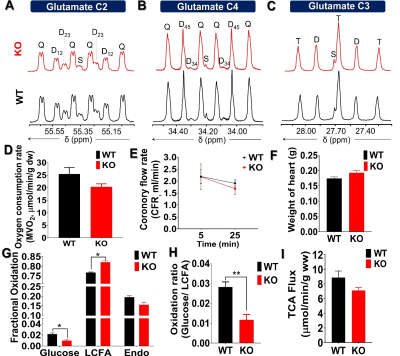
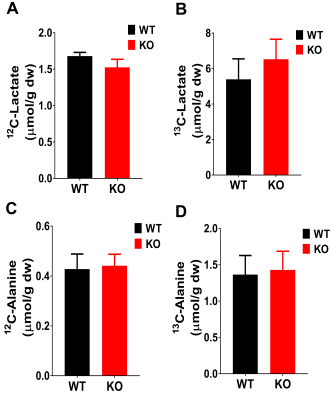
The metabolic scheme of 13C-enriched substrates provided in the perfusate was represented in Fig. 1. The substrate oxidation was assessed by analyzing 13C-multiplets from glutamate C2 (Fig. 2A), C4 (Fig. 2B), and C3 (Fig. 2C). With this labeling pattern, the C4 singlet and doublet (C4D34) arise solely from glucose oxidation. The C4 doublet due to J45 and the doublet of doublets due to J34 and J45 arise exclusively from oxidation of long-chain fatty acids. In the KO animals compared to control, the fraction of acetyl-CoA derived from long chain fatty acids increased slightly, oxidation of glucose decreased slightly, and there was little effect of mobilization of endogenous substrates for energy production. The oxygen consumption of ACC WT hearts (25.4 ± 2.6 mol/min/g dw) was not statistically different as compared to KO hearts (20.3 ± 1.1 mol/min/g dw) in metabolic steady state (Fig. 2D). After 5 min of perfusion, the coronary flow rate (Fig. 2E) was similar in WT and KO hearts, although it slightly reduced when perfusion approached the steady state (25 min). The weight of KO hearts (0.19 g ± 0.01 g) is similar to that of WT hearts (0.17g ± 0.01 g) (Fig. 2F). When compared to WT hearts, 13C NMR isotopomer analysis of heart extracts from KO mice indicated a ~7% increase in LCFA oxidation and a commensurate decrease in glucose oxidation (Fig. 2G). When expressed as a ratio of glucose/LCFA oxidation, in comparison to WT hearts, KO showed a 58.5% decrease in glucose/LCFA oxidation (Fig. 2H). TCA cycle flux (Fig. 2I) in KO hearts (7.2 ± 0.4 mol/min/g dw) did not differ substantially from that in control hearts (8.90±0.95 mol/min/g dw). The tissue concentrations of 12C-lactate (Fig. 3A) were no different in KO (1.52 ± 0.11mol/g dw) than in WT hearts (1.68 ± 0.04 mol/g dw). Similarly, 13C-enriched lactate concentrations in KO (6.52 ±1.53 mol/g dw) and WT hearts (5.39 ± 1.13 mol/g dw) were not statistically different. Tissue concentrations of endogenous (Fig. 3C) and 13C labeled alanine showed no change in accordance with these concentrations (Fig. 3D). These data indicate that glycogen mobilization was not different in hearts deficient in ACC-1 and ACC-2.CONCLUSION
We found that the ACC deficient hearts oxidized slightly more fatty acids, glucose oxidation was correspondingly reduced, TCA cycle flux was not significantly altered, and mobilization of endogenous sources was not significantly altered.Acknowledgements
This work was supported by grants from the American Heart Association (18POST34050049 to G.S.) and NIH (R37-HL034557 to A.D.S., P41-EB015908 to C.R.M., R01- EB027698 to C.K. and P01-HL020948 to J.D.H.)References
1. Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111(6):728-738. doi:10.1161/CIRCRES AHA.112.268128
2. Essop MF, Camp HS, Choi CS, Sharma S, Fryer RM, Reinhart GA, Guthrie PH, Bentebibel A, Gu Z, Shulman GI, Taegtmeyer H, Wakil SJ, Abu-Elheiga L. Reduced heart size and increased myocardial fuel substrate oxidation in ACC2 mutant mice. Am J Physiol Heart Circ Physiol. 2008 Jul; 295(1): H256-65. doi: 10.1152 / ajpheart.91489.2007. Epub 2008 May 16. PMID: 18487439; PMCID: PMC2494759.
Figures