4916
A Comparison of the Magnetic Force of MR-Conditional Cardiac Implants
Mabel Shehada1, Emile Shehada2, Ramez Shehada3, and David Prutchi4
1UCSD, San Diego, CA, United States, 2Yale University, New haven, CT, United States, 3Medical Technology Laboratories, La Mirada, CA, United States, 4Impulse Dynamics, MARLTON, NJ, United States
1UCSD, San Diego, CA, United States, 2Yale University, New haven, CT, United States, 3Medical Technology Laboratories, La Mirada, CA, United States, 4Impulse Dynamics, MARLTON, NJ, United States
Synopsis
For implant systems composed of an implantable pulse generator (IPG) and lead(s), the MR-Conditional labeling specifies a magnetic spatial gradient for the whole system but not for its individual components. To identify the component experiencing the highest magnetic force, thus dictating the safe limit, we measured multiple IPGs and leads per ASTM F2052-15. The highest force on leads under 4.77T/m, 20T/m, and 50T/m was 0.003N, 0.021N, and 0.053N, respectively. The lowest IPG forces were 0.080N, 0.529N, and 1.322N. For any magnetic spatial gradient, the lowest IPG force was 2,480% greater than the highest lead force.
Introduction and Background
Implantable medical devices in the magnetic resonance (MR) environment experience a magnetic force that may displace the implant inside the body or even tear it out of a fresh surgical wound, which is a patient safety hazard. The magnitude of the magnetic force depends primarily on the materials in the implant and the spatial gradient of the static magnetic field of the MR scanner. Since the former is fixed for a specific implant, the MR-Conditional labeling of the implant specifies a limit on the magnetic spatial gradient to ensure that the magnetic force does not exceed a safe magnitude and harm the patient.For cardiac active implantable systems composed of an implantable pulse generator (IPG) and lead(s), the MR-Conditional labeling specifies a magnetic spatial gradient for the whole system but not for its individual components. In certain clinical situations, the IPG of an implanted MR-Conditional system may be replaced with a different IPG model while keeping the original leads intact thereby creating a new mixed-component system with an unknown magnetic spatial gradient limit. Accordingly, it would be beneficial to identify the system component (i.e. the IPG or lead) experiencing the highest magnetic force and consequently dictating the safe magnetic spatial gradient limit specified in the MR-Conditional labeling.
Objective
To identify the system component dictating the safe magnetic spatial gradient limit, we investigated the magnetic force experienced by IPGs and leads of several MR-Conditional cardiac rhythm management systems from different manufacturers.Methods
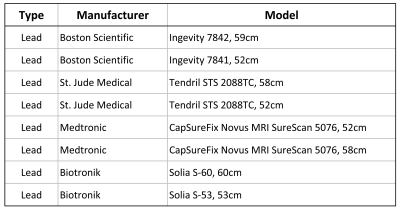
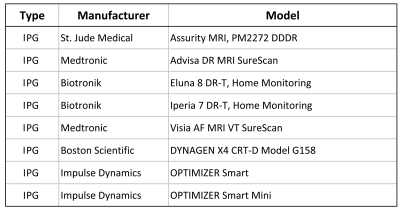
The magnetic force of sixteen MR-Conditional cardiac implants of various models made by different manufacturers was measured in a 3T MRI scanner (GE Signa HDxt 3.0T horizontal closed-bore) according to the method described in the ASTM F2052-151 standard. The cardiac implants under test were 8 leads and 8 IPGs as listed in the tables of Figures 1 and 2, respectively. The magnetic spatial gradient was measured at the test location using a gaussmeter and found to be 4.77 T/m. The measured magnetic force of each implant was scaled to higher magnetic spatial gradients of 20 T/m and 50 T/m using the formulas provided by the ASTM F2052-151 standard.Results
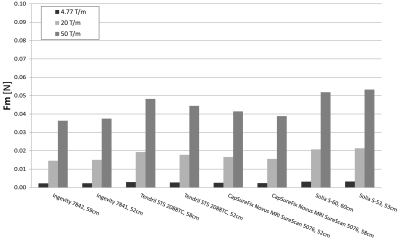
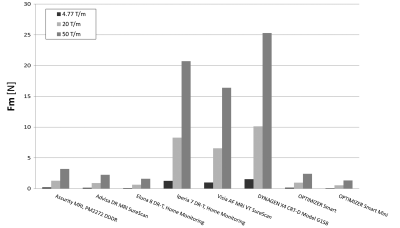
The magnetic force of the tested leads and IPGs is shown in Figures 3 and 4, respectively, under spatial magnetic gradients of 4.77 T/m, 20 T/m, and 50 T/m. The highest magnetic force of the leads under spatial magnetic gradients of 4.77 T/m, 20 T/m, and 50 T/m was 0.003 N, 0.021 N, and 0.053 N, respectively. The lowest magnetic force of the IPGs under spatial magnetic gradients of 4.77 T/m, 20 T/m, and 50 T/m was 0.080 N, 0.529 N, and 1.322 N, respectively. At any given magnetic spatial gradient, the lowest magnetic force experienced by the IPGs was 2,480% greater than the highest magnetic force experienced by the leads.Conclusions
The results above indicate that that under the same magnetic spatial gradient, the lowest magnetic force of the IPGs is significantly greater than the highest magnetic force of the associated leads. Accordingly, we conclude that IPGs experience the worst-case magnetic force relative to leads and therefore dictate the safe magnetic spatial gradient limit specified in the MR-Conditional labeling of implantable cardiac systems.Acknowledgements
This work was supported by Impulse Dynamics (USA) Inc.References
1. TM F2052−15, “Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment,” 2015.
DOI: https://doi.org/10.58530/2022/4916