4913
Evaluation of Sex and Age Differences in Myocardial Microstructure using Cardiac Diffusion Tensor Imaging1Cardiovascular Research Center, Massachusetts General Hospital, Charlestown, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Division of Health Science Technology, Harvard-Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Sexes and ages are known factors that contribute to cardiovascular pathophysiological variances and prognosis. This study used in-vivo cardiac diffusion tensor MRI (DT-MRI) to characterize how myocardial microstructure differs across sexes and ages. To that purpose, thirty healthy volunteers (14 female) were imaged with in-vivo cardiac DT-MRI and common DT-MRI parameters (MD, FA, HAT, E2A) were computed within the left ventricle (LV). No significant sex-related differences (11%, 9%, 3%, 9% respectively for MD, FA, HAT, E2A at septal regions) or age-related correlations were found. Taken together, sex and age had no effects on the quantification of in-vivo cardiac DT-MRI parameters.
Introduction
Male and female have been found to display differences in their cardiovascular anatomy and physiology, including differences in their cellular composition. Previous studies have reported differences in ejection fraction, LV mass and LV volume etc.1. These may contribute to variations in cardiovascular disease phenotypes, prognosis and treatment. Similarly, cardiac anatomy has also been found to change with age. For example, large arteries thicken due to collagen and calcium deposition as age increases2. Moreover, cellular hypertrophy causing concentric wall thickening was also observed in left interventricular septum in older adults2. However, current studies have focused on macrostructural differences across sex and age, but none have investigated the similarities or differences in microstructure. Specifically, the heart muscle has long been known to have a well-organized helical microstructure that is changed in diseased patients3. Therefore, cardiac microstructure characterization may provide additional insight and guidance regarding sex and age-specific treatment and diagnosis. Cardiac DT-MRI can be used to non-invasively evaluate the microstructure of the heart and can provide unique information about the overall organization of the helicity of the myocardium without the administration of exogenous contrast agents and exposure of radiation3,4. The goal of this study is to use cardiac DT-MRI to characterize the cardiac microstructure in male and female healthy subjects across various age groups and identify potential microstructural differences between sexes and ages.Methods
Thirty healthy volunteers (mean age 36.7 years, 14 female), recruited with approval from the institutional review board of the Massachusetts General Hospital were scanned under free breathing with a 2nd order motion compensated DWI scheme and 2DRF zoomed EPI in a 3T MAGNETOM Prisma (Siemens, Erlangen, Germany). The DWI sequence consisted of 2 b-values (b= 50/500 s/mm2) acquired over 30 directions with x2 averages for the b500 images, TR/TE=540/79ms, BW=2380Hz/pixel, resolution 2.5x2.5x8mm3, 12-15 slices to cover the entire left-ventricle (LV). Each slice was registered to its reference at the end-expiration respiratory phase using a motion compensation approach that uses respiratory binning 5,6 and the DT-MRI model was fit to obtain mean diffusivity (MD), fractional anisotropy (FA), helix angle. The helix angle transmurality (HAT) and 2nd eigenvalue angle (E2A) were then computed using manually segmented LV masks. Afterwards, the FA, MD, HAT and E2A values were computed per each subject as the median value within two regions of interest on septal and lateral regions of the heart. Statistical analysis was performed with GraphPad Prism (v 9.2.0); differences between two sex groups were assessed via two-way ANOVA tests using both regions of interest (septal vs lateral) and correlations between the DT-MRI parameters and age were assessed using linear regression analysis. Significance threshold for each analysis was determined at 0.05.Results
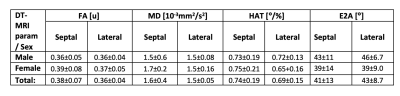
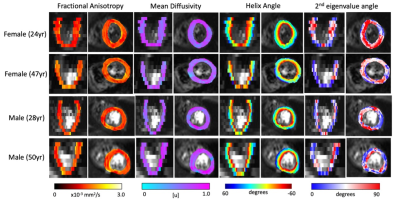
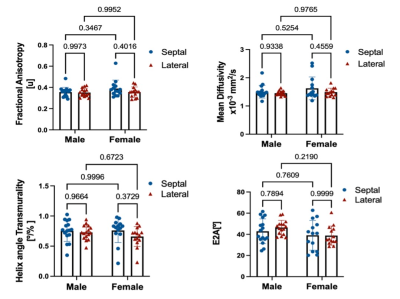
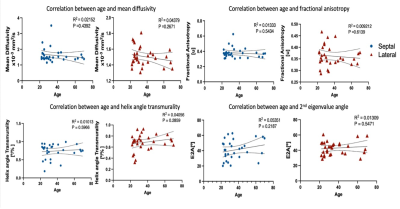
Both male and female subjects showed similar spatial distribution of the DT-MRI parameters (FA, MD, HAT and E2A), illustrated in Figure 1 for one female and one male example. The numerical values for all parameters are shown in the box-plots of Figure 2 and reported as mean and standard deviation in Table 1. Male subjects presented a FA 0.36±0.05, MD 1.5±0.61 x10-3 mm2/s2, HAT 0.73±0.19 deg/% and E2A 43±11 degrees for septum, and FA 0.36±0.04, MD 1.5±0.08 x10-3 mm2/s2, HAT 0.72±0.13 deg/% and E2A 46±6.7 degrees for lateral region. Similarly, female subjects presented an average FA 0.39±0.08, MD 1.7±0.24 x10-3 mm2/s2, HAT 0.75±0.21 deg/% and E2A 39±14 degrees for septum, and FA 0.37±0.05, MD 1.5±0.16 x10-3 mm2/s2, HAT 0.65+0.16 deg/% and E2A 39±9.0 degrees for lateral region. No statistically significant differences were identified by the two-way ANOVA tests (Figure 2). Regression analysis of the four DT-MRI parameters against age are illustrated in Figure 3. The resulting R2 values were 0.013, 0.022, 0.010, and 0.054 for FA, MD, HAT and E2A for septal region, respectively, and 0.009, 0.044, 0.041, and 0.013 respectively for lateral region, although these were not statistically significant (P values were 0.54, 0.44, 0.60 and 0.22 for septum respectively and 0.61, 0.27, 0.29 and 0.55 for lateral region respectively).Discussion
Despite previously reported macrostructural differences in cardiac anatomy, our experiments did not find any significant difference in microstructural parameters across sexes nor any correlation with age. However, the sample size for this study (30) may not be enough to differentiate potentially important differences. For example, a decrease of 5% in FA, which is similar to the difference in means between genders, has been shown to increase the likelihood of ventricular arrhythmia7.Conclusion
Cardiac DT-MRI is a feasible technique for in-vivo characterization of the microstructure of the heart. As for the result of this study, no significant differences were found between sexes nor across age in terms of FA, MD, HAT and E2A for a small cohort of healthy volunteers. Future work will further study the pathophysiological differences in cardiac microstructure with DT-MRI techniques in the context of sex and age differences.Acknowledgements
The project is funded by National Institutes of Health R01HL151704, R01HL159010, and R01HL135242.References
1. Bucciarelli-Ducci, C. et al. Cardiovascular disease in women: insights from magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance 2020 22:1 22, 1–17 (2020).
2. Fleg, J. L. & Strait, J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart failure reviews 17, 545 (2012).
3. Nguyen, C. T., Dawkins, J., Bi, X., Marbán, E. & Li, D. Diffusion Tensor Cardiac Magnetic Resonance Reveals Exosomes From Cardiosphere-Derived Cells Preserve Myocardial Fiber Architecture After Myocardial Infarction. JACC: Basic to Translational Science 3, 97–109 (2018).
4. Nguyen, C. T. et al. In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magnetic Resonance in Medicine 76, 1354–1363 (2016).
5. Nguyen, C. T. et al. Free‐breathing diffusion tensor MRI of the whole left ventricle using second‐order motion compensation and multitasking respiratory motion correction. Magnetic Resonance in Medicine 85, 2634–2648 (2021).
6. Jaume Coll-Font et al. Manifold-based respiratory phase estimation enables motion and distortion correction of free-breathing cardiac diffusion tensor MRI. Magnetic Resonance in Medicine 1–14 (2021) doi:10.1002/mrm.28972.
7. Ariga, R. et al. Identification of Myocardial Disarray in Patients With Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. Journal of the American College of Cardiology 73, 2493–2502 (2019).
Figures