4912
3D Inversion recovery-FLASH and myocardial perfusion MRI imaging in a sheep model of myocardial infarction1Physiology, University of Toronto, North York, ON, Canada, 2Early Origins of Adult Health Research Group, Health and Biomedical Innovation, University of South Australia, Adelaide, Australia, 3South Australian Health & Medical Research Institute, Preclinical, Imaging & Research Laboratories, Adelaide, Australia, 4Cardiac Imaging Research Group, Department of Heart Health, South Australian Health and Medical Research Institute, Flinders University, Adelaide, Australia, 5Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6Division of Cardiology, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
We investigated the accuracy of cardiac MRI for detecting myocardial infarction (MI) induced by coronary artery ligation surgery in a sheep model. 3D-inversion recovery (IR) FLASH sequence and first-pass perfusion imaging was feasible and provided accurate information about the size and location of MI immediately after surgery and at 15-day follow-up. The IR-FLASH sequence detected infarcts (91.2% sensitivity, 99.9% specificity) compared with gold-standard pathology staining findings. This may prove useful for preclinical research in which it is important to document the natural history of myocardial injury, such as in studies investigating interventions aimed at mitigating the long-term impact of MI.
INTRODUCTION
Ischaemia resulting from obstruction of coronary artery blood flow leads to a spectrum of myocardial injury. Accurate assessment of the size and location of a myocardial infarction (MI) is important in clinical practice and for preclinical research. A variety of MRI approaches exist for assessing MI including first pass myocardial perfusion and early and late gadolinium enhancement (GE), while refinements of these techniques such as 3D acquisitions have been shown to improve the detection of small defects such as those caused by microvascular obstruction1. In this study, our aim was to investigate the feasibility and accuracy of first-pass perfusion imaging and a 3D inversion recovery FLASH (IR-FLASH) sequence for the detection of MI in a sheep model compared with the gold-standard of conventional histopathology.METHODS
Adolescent sheep (n=12) underwent MI surgery under general anesthesia induced by ketamine (5mg/kg) and diazepam (0.3mg/kg) and maintained with isoflurane (2-3%). The second branch of the left anterior descending coronary artery was ligated to induce MI. Lambs remained anesthetized and underwent cardiac MRI (CMR) immediately after surgery (day 0) and at 7- and 15-days post-MI. An ear artery was catheterized, and the arterial pressure waveform was used for cardiac gating. Gadolinium (Gd-DTPA; 0.1 mmol/kg; intravenous; jugular vein) was administered to perform first-pass perfusion imaging and early (EGE) and late GE (LGE) imaging. First-pass perfusion imaging was performed with the following parameters: voxel size = 1.4x1.4x6.0 mm; field of view (FOV) = 306mm; flip angle = 10deg; slices = 3; repetition time (TR) = 297ms; echo time (TE) = 1.27ms; inversion time (TI) = 160ms; segments = 111; scan time = ~1minute. Mid-apical short-axis, 2 chamber(2CH), and 4 chamber (4CH) views were prescribed, targeting the expected area of injury in the anterolateral segments of the left ventricle. Following the perfusion imaging, a 3D-IR FLASH sequence with respiratory gating was employed to undertake EGE and LGE imaging with the following parameters: voxel size = 0.6x0.6x0.9mm; slices = ~80; TR = 597ms; TE = 1.27ms; TI = 450ms; segments = 45; scan time = ~8 minutes. A respiratory gating navigator was placed across the diaphragm. One day after the final scan, the animals were humanely sacrificed and the heart was dissected, stained with 2,3,5-triphenyltetrazolium chloride (TTC), and cut into ~1cm short-axis slices. Enhancements on IR-FLASH acquisitions were compared to pathological staining findings as gold-standard (Figure 1) using the American Heart Association2 (AHA) segmental nomenclature. The sensitivity and specificity of the MRI was calculated and the McNemar’s test was performed to evaluate variation in the findings of the MRI and histological methods (*P<0.05).RESULTS
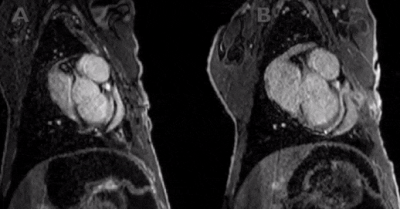
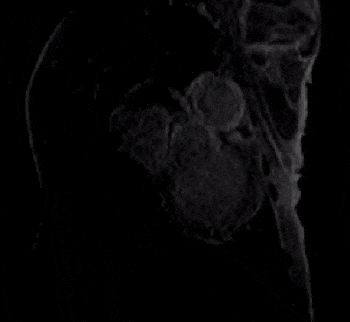
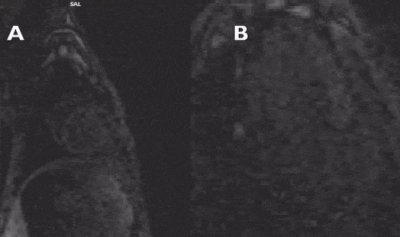
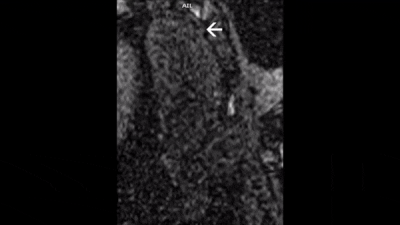
All defects were apical to the papillary muscles and the transmurality of defects could be clearly assessed in the affected slices. The most commonly injured territories were the anterior and/or antero-lateral segments of the myocardium (AHA segments= 7,12,13,16; n=10/12; Figure 2). In two cases, enhancement was also seen in inferior segments (AHA segments= 7,10,11,12,15,16; Figure 3). In all cases, diseased segments were involved across all serial imaging sessions. The infarct sizes varied over time, which was demonstrated by the IR-FLASH imaging (Figure 2). The sensitivity of IR-FLASH in detecting MI was 91.2% and specificity was 99.9% compared with the gold-standard pathological findings (P=0.625). First-pass perfusion imaging was feasible, and when the imaging was acquired in the correct imaging plane revealed ischemic regions (Figure 4 & Figure 5).CONCLUSION
The use of perfusion imaging and 3D-IR FLASH to assess the sequelae of an experimental model of MI is feasible in a sheep model. When compared with gold-standard pathological staining findings, the IR-FLASH had a sensitivity of 91.2% and specificity of 99.9% in diagnosing the regions of MI. We conclude this imaging approach is robust and allows the assessment of MI position and size, which may be useful for studies assessing the functional significance or molecular changes associated with MI. The high spatial resolution offered by our 3D LGE sequence avoids the potential for partial voluming effects and may prove especially useful in cases of smaller infarctions undetectable by conventional 2D LGE. Moreover, the application of an IR-FLASH sequence to perform EGE immediately after MI surgery, to the best of our knowledge, is novel. Our approach may prove useful in both clinical and preclinical settings for tracking the progression of MI and for risk stratification. This technique is especially useful in the setting of an acute MI, which may not be detected by LGE. First-pass perfusion imaging was less sensitive in this context, which we attribute to incomplete coverage of the ventricle resulting from the demand for high temporal resolution.Acknowledgements
The authors acknowledge the facilities and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at Preclinical Imaging and Research Laboratories, SAHMRI. The work is funded by a National Health and Medical Research Council Ideas Grant to JLM, MS, JS and CKM (APP1182242). JLM is funded by an Australian Research Council Future Fellowship (FT170100431).)References
1. Kramer, C. M. et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 22, (2020).
2. Cerqueira, M. D. et al. Standardized myocardial sementation and nomenclature for tomographic imaging of the heart: A Statement for Healthcare Professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105, 539–542 (2002).
Figures