4908
MR Multitasking-based Dynamic Imaging for Cerebrovascular Evaluation (MT-DICE): A Simulation Study for Accuracy Validation1Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Department of Neurosurgery, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 5Department of Radiation Oncology, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 6Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
MR Multitasking-based Dynamic Imaging for Cerebrovascular Evaluation (MT-DICE) technique is recently developed and has the potential to provide DCE- and leakage-corrected DSC-MRI parameters simultaneously with one 7.6-minute scan and a single-dose contrast injection. However, it remains a practical challenge to validate the technique against their respective references with repeated contrast injections in separate days. In this work, we perform a numerical simulation study to validate the accuracy of MT-DICE in the estimation of permeability and leakage-corrected perfusion metrics.
Introduction
DSC-MRI and DCE-MRI provide perfusion- and permeability-related parameters, respectively, and are evolving as increasingly common modalities for evaluating a variety of brain cancers1,2. Their different but complementary information may form a more complete basis for evaluation of the complex and heterogeneous tumor microenvironment. However, acquiring both in one exam requires two separate scans as well as two contrast injections. Recently, we developed an MR Multitasking-based Dynamic Imaging for Cerebrovascular Evaluation (MT-DICE) technique that provides DCE- and leakage-corrected DSC-MRI parameters simultaneously with one 7.6-minute scan and a single-dose contrast injection3,4. It remains a practical challenge to validate the technique against their respective references with repeated contrast injections in separate days. In this work, we perform a numerical simulation study to validate the accuracy of MT-DICE in the estimation of permeability and leakage-corrected perfusion metrics.Methods
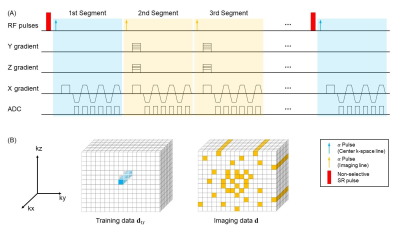
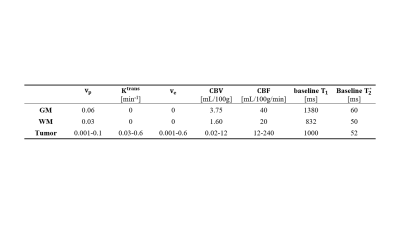
To better simulate the highly heterogeneous environment within brain tumors, a 3D anthropomorphic digital reference brain phantom incorporating a tumor model from a deidentified glioblastoma patient was created5-7. The $$${T_{1}}$$$-/$$${T_{2}^{*}}$$$-based arterial input functions (AIFs) were generated according to Jaspers et al.8 and Simpson et al.9, respectively, with realistic parameters at a 0.1-s temporal resolution. The dynamic $$${T_{1}}$$$/$$${T_{2}^{*}}$$$ curves were generated for gray matter (GM), white matter (WM) and tumor with the parameters listed in Table 1, and the residual function $$$R(t)$$$ was modeled as:$$R(t)=exp(-CBF\cdot t/CBV)$$where $$$CBF$$$ represents cerebral blood flow and $$$CBV$$$ represents cerebral blood volume. Subsequently, the simulated dynamic signal intensities were calculated based on the signal equation, which depends on our sequence design, using the downsampled dynamic $$${T_{1}}$$$/$$${T_{2}^{*}}$$$ curves at a temporal resolution of 1 s, which is consistent with the temporal resolution used in our in vivo study4. The simulated images were Fourier transformed with complex white Gaussian noise added to achieve a signal-to-noise ratio of 30. The generated k-space data were first undersampled and then reconstructed using the MT-DICE technique (Figure 1). Specifically, MT-DICE models the 6-dimensional image $$$A(x,y,z,\tau,{T_{E}},t)$$$ as a low-rank tensor $$$\mathfrak{A}=\phi \times_{1} \mathbf{U_{r}}$$$. The temporal factor tensor $$$\phi$$$ is first determined from the training data and the spatial coefficients $$$\mathbf{U_{r}}$$$ are reconstructed by fitting $$$\phi$$$ to the imaging data $$$\mathbf{d}$$$, with undersampling pattern $$$\Omega$$$, spatial encoding model $$$\mathbf{E}$$$ and regularization parameter $$$\lambda$$$ for spatial total variation penalty $$$TV(\cdot)$$$. $$\hat{\textbf{U}}_\textbf{r}=\arg \min \left \| \textbf{d}-\Omega (\phi \times_{1} \textbf{E}\textbf{U}_{\textbf{r}}) \right \|_{2}^{2}+\lambda TV(\textbf{U}_{\textbf{r}})$$Dynamic $$${T_{1}}$$$/$$${T_{2}^{*}}$$$ fitting and kinetic modeling were performed on all slices involving the tumor. The derived permeability metrics were adopted to perform leakage correction for the estimations of DSC-MRI metrics based on a combined biophysical and pharmacokinetic approach10. In addition to the leakage-corrected perfusion parameters, the non-leakage-corrected perfusion metrics were derived in the conventional way11.Results
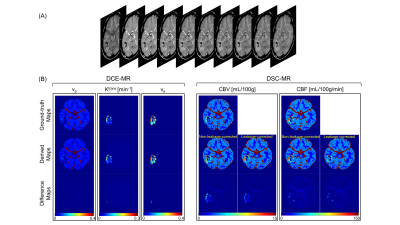
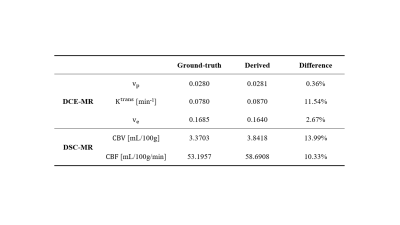
The simulated dynamic image series of one representative slice from the digital brain phantom are displayed in Figure 2A. The ground-truth and MT-DICE derived maps of DCE-MRI and DSC-MRI parameters as well as their differences are shown in Figure 2B. MT-DICE was capable of correcting contrast leakage effects, which is more evident in $$$CBV$$$ quantification, thus leading to smaller percentage errors compared with the non-leakage-corrected counterpart (13.99% vs. 18.87%). Table 2 summarizes the quantitative results measured from the entire tumor model except the necrotic core.Discussion and Conclusion
In this work, a simulation study was performed to validate the accuracy of MT-DICE in the estimation of permeability and leakage-corrected perfusion metrics. In the field of DCE-MRI and DSC-MRI, the common method of assessing bias and variance resulting from an algorithm is through the development and application of a grid-based digital reference objects (DRO). To better simulate the highly heterogeneous environment within brain tumors, a 3D anthropomorphic digital reference brain phantom incorporating a tumor model was adopted, which is more sophisticated and accurate. The simulation results validated the accuracy of the proposed MT-DICE in kinetic parameter estimation.Acknowledgements
No acknowledgement found.References
1. Essig M, Nguyen TB, Shiroishi MS, Saake M, Provenzale JM, Enterline DS, Anzalone N, Dörfler A, Rovira À, Wintermark M. Perfusion MRI: the five most frequently asked clinical questions. American Journal of Roentgenology 2013;201(3):W495-W510.
2. Bergamino M, Bonzano L, Levrero F, Mancardi G, Roccatagliata L. A review of technical aspects of T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in human brain tumors. Physica Medica 2014;30(6):635-643.
3. Hu Z, Christodoulou A, Wang N, Xie Y, Sun B, Bi X, Han F, Song S, Maya M, Li D, Fan Z. MR multitasking-based dynamic imaging for cerebrovascular evaluation (MT-DICE): development and feasibility study. Proceeding International Society of Magnetic Resonance in Medicine 2020; 1827.
4. Hu Z, Christodoulou A, Wang N, Xie Y, Cao T, Maya M, Yang W, Li D, Fan Z. MR multitasking-based dynamic imaging for cerebrovascular evaluation (MT-DICE): further development and feasibility study on brain cancer. Proceeding International Society of Magnetic Resonance in Medicine 2021; 0048.
5. Bosca RJ, Jackson EF. Creating an anthropomorphic digital MR phantom—an extensible tool for comparing and evaluating quantitative imaging algorithms. Physics in Medicine & Biology 2016;61(2):974.
6. Cocosco CA, Kollokian V, Kwan RK-S, Pike GB, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. 1997. Citeseer.
7. Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE transactions on medical imaging 1998;17(3):463-468.
8. Jaspers K, Aerts HJ, Leiner T, Oostendorp M, van Riel NA, Post MJ, Backes WH. Reliability of pharmacokinetic parameters: Small vs. medium‐sized contrast agents. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2009;62(3):779-787.
9. Simpson NE, He Z, Evelhoch JL. Deuterium NMR tissue perfusion measurements using the tracer uptake approach: I. Optimization of methods. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 1999;42(1):42-52.
10. Stokes AM, Semmineh N, Quarles CC. Validation of a T1 and leakage correction method based on multiecho dynamic susceptibility contrast MRI using MION as a reference standard. Magnetic resonance in medicine 2016;76(2):613-625.
11. Straka M, Albers GW, Bammer R. Real‐time diffusion‐perfusion mismatch analysis in acute stroke. Journal of Magnetic Resonance Imaging 2010;32(5):1024-1037.
Figures