4901
Evaluation of a Novel Manganese-Based Contrast Agent for Cardiac MRI in the Setting of Myocardial Ischemia-Reperfusion1Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 3Louisiana State University Health Sciences Center, New Orleans, New Orleans, LA, United States, 4Harvard Medical School, Boston, MA, United States, 5Division of Health Science Technology, Harvard-Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Cardiac MRI a powerful imaging modality to assess cardiac anatomy and function, and its utility can be greatly enhanced with the use of contrast agents. Conventional contrast agents are nephrotoxic which limits their utility. We evaluate a novel contrast agent which is safe for use in renal dysfunction. We compare its performance against that of a conventional agent a porcine model of ischemia-reperfusion. This novel contrast agent demonstrates equivalent performance in assessing myocardial fibrotic scars, indicating its potential clinical use in the workout of cardiac patients with renal impairment.
Introduction
Cardiac MRI (cMRI) is a rapidly growing field within cardiac imaging. The recent MR-INFORM and SCD-HeFT clinical trials demonstrated cMRI with contrast to be equivalent to invasive cardiac catheterization in terms of prognostic value in the setting of myocardial infarction1. Recent advancements in image acquisition and processing techniques have contributed to the rising popularity of cMRI by attenuating previous burdens such as motion distortion and breath-hold requirements. Contrast agents (CAs) can greatly enhance the utility of cMRI and provide greater clarity in evaluating anatomical changes such as fibrotic scars in the setting of ischemia-reperfusion (IR). Conventional CAs for MRI and CT are Gadolinium-based and heavily iodinated compounds respectively. Both are often contraindicated in patients with renal dysfunction. The recent consensus statement by the American College of Radiology discourages their use in patients with a glomerular filtration rate < 30 mL/min/1.73m2. This is particularly problematic in the workout of cardiac patients where the coincidence of renal impairment is approximately 30%3.We here evaluate a novel manganese-based CA, RVP-001. RVP-001 was designed to undergo partial hepatobiliary excretion for safety in patients with renal dysfunction4. We compare the performance of RVP-001 with that of gadoteric acid (Gd-DOTA), a conventional GBCA. We assess the performance of the two CAs in several metrics including gross scar volume, transmurality and intra-cardiac localization of the scar, and washout characteristics.
Methods
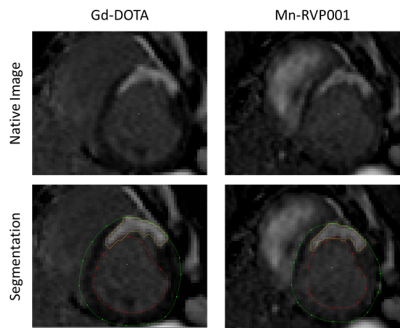
We compare the performance of RVP-001 to gadoteric acid (Gd-DOTA) in a porcine model of myocardial ischemia-reperfusion (MIR). All experiments were performed under approval of the Institutional Animal Care and Use Committee of the Massachusetts General Hospital. MIR was induced for 80 minutes via trans-carotid catherization and occlusion of the left anterior descending artery distal to the second diagonal branch. Evaluation of scar size was performed three days post-MIR with both Mn-RVP001 and Gd-DOTA in a 3T MAGNETOM Prisma and a 3T Biograph mMR (Siemens, Erlangen, Germany). The imaging sequence consisted in delayed enhanced T1 weighted images captured at 5, 10, and 15 minutes after contrast injection. These images were acquired with a stack of 2D bSSFP images to cover the entire LV (TR/TE=739/1.1ms, BW=1184Hz/pixel, resolution 1.8x1.8x8mm3, 12-15 slices). The second contrast agent was injected 180 +/- 30 min after the first injection to allow for washout of the first contrast agent. During the washout period, T1 mapping was performed repeatedly to assess the level of CA still present in the body and to characterize the recovery periods of the two CAs. The order of the contrast agents was randomized across pigs to avoid bias introduced by contrast remaining in the tissue. Quantification of scar volume was performed semi-automatically using Segment. Statistical analyses were performed using MATLAB and Prism. Statistical significance was assessed with Wilcoxon signed-rank test (significance level p=0.05). Triphenyl tetrazolium chloride (TTC) staining will be performed to histologically validate CA performance.Results
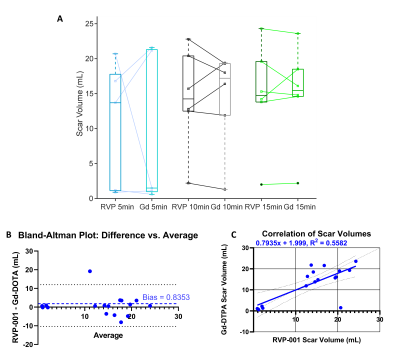
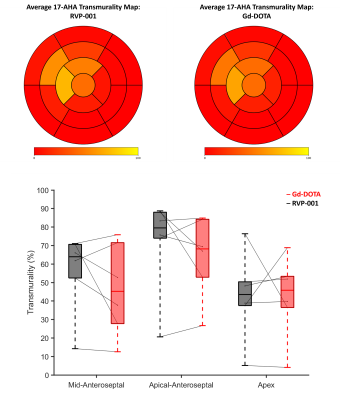
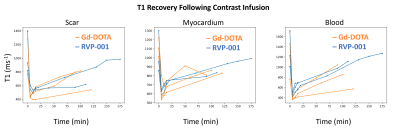
Wilcoxon-signed rank tests were conducted to compare the reported scar volumes from the two CAs (N = 6) at 5-, 10-, and 15-minutes post-contrast injection. No significant differences between CAs were observed (P-values at the three timepoints were 0.77, 0.99, and 0.93 respectively). Bland-Altman analysis reveals a bias of 0.8353. The Pearson-Correlation Coefficient between the two CAs was found to be 0.56 (Figure 2). The standardized AHA 17-segment model was applied to quantify scar positioning and transmurality. Scar transmurality was described as a percentage of total myocardial thickness in a line-based approach. Wilcoxon-signed rank tests revealed no significant differences in scar localization/transmurality between the two agents (Figure 3). The washout pharmacokinetics of the two agents (NGd = 3; NMn = 3) was compared using T1 recovery mapping (Figure 4). Recovery curves were fitted with a sigmoid function (S0 / [1 + exp(-t/2β)]). The recovery time constant, β, was calculated; median time constants for each CA were βMn = 44.93, βGd = 43.26.Discussion
We find that RVP-001 accurately replicates the function of Gd-DOTA in the setting of MIR. Macroscopically, RVP-001 produces similar scar volumes to Gd-DOTA post IR. This similarity is maintained when finer anatomical regions of interest are considered. The performance of RVP-001 is maintained across a wide range of fibrotic burdens and demonstrates nominal bias relative to the conventional agent. Furthermore, the washout properties of Mn-RVP001 closely approximate those of Gd-DOTA and do no incur any additional cost in return to baseline.Conclusion
RVP-001 recapitulates the tissue characterization properties of Gd-DOTA in the setting of myocardial IR including gross scar volume and transmurality. RVP-001 demonstrates close correlation with and nominal bias relative to Gd-DOTA. These findings suggest that RVP-001 may be a clinically viable alternative to conventional GBCAs in cMRI.Acknowledgements
Research in this publication was supported by R01HL151704, R01HL159010, R01HL135242.
Additional support was provided by R43HL156713 SBIR with Reveal Pharmaceuticals.
References
1) Nagel, E., Greenwood, J. P., McCann, G. P., Bettencourt, N., Shah, A. M., Hussain, S. T., Perera, D., Plein, S., Bucciarelli-Ducci, C., Paul, M., Westwood, M. A., Marber, M., Richter, W.-S., Puntmann, V. O., Schwenke, C., Schulz-Menger, J., Das, R., Wong, J., Hausenloy, D. J., … Berry, C. (2019). Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. New England Journal of Medicine, 380(25), 2418–2428. https://doi.org/10.1056/nejmoa1716734
2) Weinreb, J. C., Rodby, R. A., Yee, J., Wang, C. L., Fine, D., McDonald, R. J., Perazella, M. A., Dillman, J. R., & Davenport, M. S. (2021). Use of intravenous gadolinium-based contrast media in patients with kidney disease: Consensus statements from the American college of radiology and the national kidney foundation. In Radiology (Vol. 298, Issue 1, pp. 28–35). Radiological Society of North America Inc. https://doi.org/10.1148/RADIOL.2020202903
3) Foley, R. N., Murray, A. M., Li, S., Herzog, C. A., McBean, A. M., Eggers, P. W., & Collins, A. J. (2005). Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States medicare population, 1998 to 1999. Journal of the American Society of Nephrology, 16(2), 489–495. https://doi.org/10.1681/ASN.2004030203
4) Gale, E. M., Atanasova, I. P., Blasi, F., Ay, I., & Caravan, P. (2015). A Manganese Alternative to Gadolinium for MRI Contrast. Journal of the American Chemical Society, 137(49), 15548–15557. https://doi.org/10.1021/jacs.5b10748
Figures