4899
Dynamic 3D T1 MRI method for T1-based Quantification of DCE for the Measurements of kinetic Parameters on Carotid Atherosclerotic Plaques1UCAIR, Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Department of Surgery, University of Utah, Salt Lake City, UT, United States, 3VASLCHCS, Salt Lake City, UT, United States
Synopsis
This work introduced a reliable, comprehensive carotid DCE technique, including a high spatial and temporal resolution 3D dynamic T1 sequence and retrospective reconstruction methods to measure kinetic parameters on carotid atherosclerotic plaque. This technique can be used to evaluate the correlation between kinetic parameters and ischemic stroke. We believe that plaque kinetic parameters can be used in subsequent clinical trials as a changeable predictor of ischemic stroke risk. Clinicians could then use carotid DCE to monitor early response to medical therapy, and thereby improve detection and treatment of unstable carotid plaque and prevention of stroke.
PURPOSE
Carotid atherosclerosis is one of the most common causes of ischemic stroke(1). In histologic studies, contrast leakage may result from endothelial dysfunction or be secondary to intraplaque inflammation and adventitial neovessel rupture(2). While dynamic contrast‐enhanced (DCE) MRI has shown potential in quantitative assessment of the neovascular architecture and perfusion properties in the carotid artery wall(3), its application to vessel walls still faces demanding technical challenges(4). In model-based analysis of DCE, nonlinearity between T1w-DCE signal intensity and contrast agent (CA) concentration, particularly during peak enhancement, introduces potential errors in arterial input function (AIF) calculation and tissue CA concentration(5). Although CA concentration can be directly obtained [DP1] from quantitative measurements of the longitudinal relaxation rate(6), current variable flip angle (VFA) T1 methods(7) are not suitable for dynamic T1 measurements at high temporal resolution. We have developed a mono FA T1 approach which acquires a single reference (sr) image using a lower FA before the start of the dynamic imaging, and acquires images with a higher FA dynamically(8). In this work, we introduce a dynamic sr-VFA T1 MRI method capable of T1-based quantification of CA concentration at high temporal resolution to measure the accurate kinetic parameters on carotid atherosclerotic plaques.METHODS
A 3D Stack of Star (SOS) spoiled gradient echo sequence was modified with a pseudo golden angle (PGA) view increment to allow retrospective reconstruction. The PGA increment was based on a ratio of 233 and 377 and caused the k-space trajectory to repeat exactly after a 377 of projections. To increase the temporal resolution, we used an asymmetric k-space weighted image contrast (KWIC) reconstruction(9) with 377 total and 29 innermost projections (13 times acceleration). T1 maps were estimated dynamically using a recently developed srVFA approach. The optimal choice of reference FA was 5° after Monte Carlo simulation. All scans were performed on a 3T MRI system with composite head and neck coils. A uniform phantom with known T1 value was scanned. With IRB consent, we performed the srVFL T1 study on four patients with carotid disease. One reference FA data was acquired with 377 projections and FA=5O before contrast administration. Dynamic data parameters were: 0.7 mm isotropic dimension, 24 slices, FA=15O, TE/TR = 2.46/5.62ms, 3016 projections. Dynamic T1 maps were computed using a KWIC recon giving an effective temporal resolution of 2.92 s and sr-VFA technique. The B1 maps was used to correct T1 map calculations. After computing T1 maps, corresponding dynamic concentration maps (C(t)) were calculated using (T1(t))-1 = (T1(0))-1 +r1(C(t)), where r1 is the specific relaxivity of prohance on 3T(3,41s-1mM-1)(10). All computations including reconstruction were done offline with custom written Matlab codes. Two compartment kinetic model(11) was fitting to plaque and blood CA concentration time curves.RESULTS
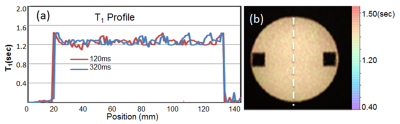
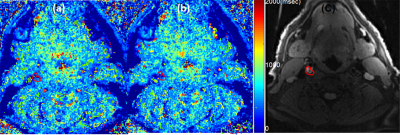
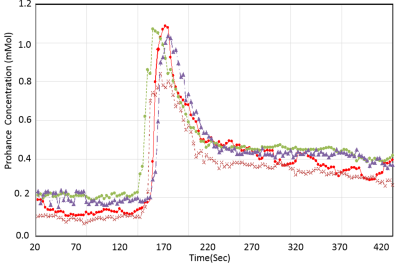
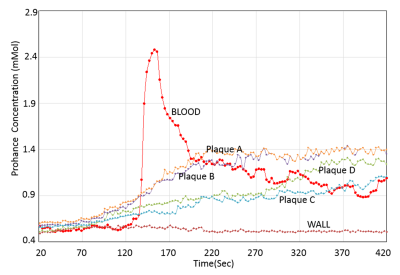
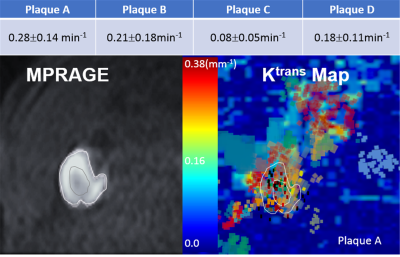
The phantom study results are shown in the Fig 1. The dynamic T1 profiles(a) and T1 map(b) demonstrate the relatively uniform T1 distribution across the phantom matched to the true T1. Fig 2 shows the dynamic T1 maps of a patient with carotid atherosclerotic plaque at 10sec (a), 350sec (b) and T1-weighted image. The T1 value of the plaque was deceased from 570±84 ms (red contour in (a)) to 275±48ms (b). The blood CA concentration curves from four patients are plotted on Fig 3. The dynamic CA concentration changes on blood, wall (computed from one patient data), and four different carotid atherosclerotic plaques are plotted on Fig 4. The blood concentration curve was scaled by 2 times of the original concentration. The dynamic concentration curves indicate the more active contrast uptake within the plaque A and B compared to plaque C. There was no indication of CA uptake in the vessel wall. A table on Fig 5 are summarized the mean Ktans within four carotid plaques. Fig 4 also demonstrates a Ktrans map of intraplaque hemorrhage (IPH) found on a MPRAGE image of plaque A. The mean Ktrans within a white ROI was 0.28±0.14min-1, while the mean value of Ktran within the plaque C was 0.18 ±0.11 min-1.DISCUSSION and CONCLUSION
The precision of the T1 measurement of proposed technique can be optimized with proper choice and accurate estimation of FAs. Using a VFA method will amplify noise through its nonlinear nature of calculating T1. For standard VFA, the ideal choice of FA will produce ~71% of Ernst angle signal with the two FA on different sides of the Ernst angle. A more accurate estimate of the percent of the Ernst angle signal could be simulated with much finer FA increments, or possibly an exact estimate could be derived(12). Further works to estimate the actual FAs of proposed technique are warranted. Also, during the dynamic process, blood flowing through imaging slab will not reach steady state, requiring additional modeling to extract the T1 of blood acquired using VFA technique. Contrast concentration determined from these dynamic T1 measurements during the enhancement process has a potential to provide more accurate kinetic quantification. Our method will improve the identification and measurement of carotid microvascular alterations, which could represent a modifiable risk factor for carotid atherosclerosis. In future work, we will evaluate and compare the accuracy of dynamic T1 measurements and the repeatability of kinetic quantification of the proposed technique.Acknowledgements
Supported by R01 HL127582, RSNA Research Scholar Grant RSCH1414, AHA Scientist Development Grant 17SDG33460420, Siemens Medical Solutions, the Clinical Merit Review Grant from the Veterans Administration health Care System.References
1. Moody AR, Murphy RE, Morgan PS, et al.: Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003; 107:3047–3052.
2. Saam T, Hatsukami TS, Takaya N, et al.: The vulnerable, or high-risk, atherosclerotic plaque: Noninvasive MR imaging for characterization and assessment. Radiology 2007:64–77.
3. Gaens ME, Backes WH, Rozel S, et al.: Dynamic Contrast-enhanced MR Imaging of Carotid Atherosclerotic Plaque: Model Selection, Reproducibility, and Validation. Radiology 2013; 266:271–279.
4. Wang N, Christodoulou AG, Xie Y, et al.: Quantitative 3D dynamic contrast‐enhanced (DCE) MR imaging of carotid vessel wall by fast T1 mapping using Multitasking. Magn Reson Med 2019; 81:2302–2314.
5. Calcagno C, Mani V, Ramachandran S, Fayad ZA: Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis 2010; 13:87–99.
6. Taheri S, Gasparovic C, Shah NJ, Rosenberg GA: Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med 2011; 65:1036–1042.
7. Cheng H-LM, Wright GA: Rapid high-resolution T1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med 2006; 55:566–574.
8. Svedin BT, Payne A, Bolster BD, Parker DL: Multiecho pseudo-golden angle stack of stars thermometry with high spatial and temporal resolution using k-space weighted image contrast. Magn Reson Med 2018; 79:1407–1419.
9. Song HK, Dougherty L: Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med 2004; 52:815–24.
10. Shen Y, Goerner FL, Snyder C, et al.: T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7T. Invest Radiol 2015; 50:330–338.
11. Tofts PS, Brix G, Buckley DL, et al.: Estimating kinetic parameters from dynamic contrast-enhanced T1- weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging 1999:223–232.
12. Schabel MC, Morrell GR: Uncertainty in T(1) mapping using the variable flip angle method with two flip angles. Phys Med Biol 2009; 54.
Figures