4897
Carotid atherosclerotic plaque segmentation in multi-weighted MRI using a two-stage neural network model1Mallinckrodt Institute of Radiology, Washington University in St. Louis, St. Louis, MO, United States, 2Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Our objective of this study is to accurately segment and classify carotid atherosclerotic plaque components in a completely automated manner, based on multi-weighted MRI. Specifically, we segmented each pixel using a two-module neural network model. Furthermore, we generated segmentation uncertainty maps with a Bayesian method to evaluate the inherent uncertainty of this segmentation task.
Purpose
Atherosclerotic plaque is one of the most common subtypes of vascular disease, and rupture of an atherosclerotic plaque in the carotid artery could lead to stroke. Identification of carotid atherosclerotic plaque components using multi-weighted magnetic resonance images (MRI) is being investigated to provide an efficient and reliable contribution to stroke risk assessment. However, manually conducting such identification is expensive, time-consuming, tedious, and suffers from significant intra-reader variability. To address the challenges, we propose a two-module deep-learning-based approach that classifies carotid plaque components reliably and reproducibly. A key novelty is the ability of the proposed method to use ex vivo high-resolution MRI carotid plaque images and corresponding histopathological slides during the training process.Methods
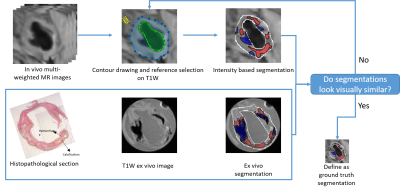
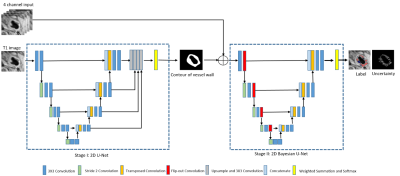
MRI data collection: Nine patients with carotid stenosis confirmed by the echo exam underwent carotid MRI first, followed by endarterectomy (within one week of the MRI exam). The ex vivo MRI of carotid plaque tissue was performed within 30 min after surgery and the tissue was then fixed in formalin for pathological staining (H&E and Mason). Multi-weighted MR images were obtained, including T1 weighted, T2 weighted, proton-density weighted, and time of flight (TOF) images for all in vivo and ex vivo MR plaque images, co-registered based on the distance to the bifurcation. The ex vivo data sets ($$$0.098\times0.098\times1 mm^3$$$) were acquired on a 3T Siemens Allegra system, whereas the in vivo MRI data sets ($$$0.47\times0.47\times3 mm^3$$$) were acquired at a 1.5T Sonata system. A total of 84 slices (in vivo, ex vivo, and histopathology) were collected. The in vivo data was preprocessed with coil inhomogeneity correction, denoising, and motion correction1-3. The plaque components in in vivo (lipid-rich necrotic core (LRNC) with and without hemorrhage, calcification, and fibrosis) were first classified based on an established intensity method, relative to the adjacent sternocleidomastoid muscle4. The ground truth plaque component boundaries were then determined by comparison to plaque components determined from the high-resolution ex vivo MRI plaque images and histopathology with adjustment of reference intensity.Proposed method: We had a limited training data size. Further, in the process of reference muscle tissue selection during ground truth generation, there was a certain degree of randomness. To address this challenge, we recognized that recent research on Bayesian neural networks (BNNs) demonstrates the ability to estimate uncertainty and solve problems in domains where data is scarce5. Taking advantage of this fact, we developed a deep learning-based method that consisted of two networks in sequence, a convolutional neural network (CNN) followed by a BNN. The goal of the CNN was to segment the contours of lumen and outer artery wall with input T1W images, while the goal of the BNN was to classify carotid plaque and estimate the aleatoric and epistemic uncertainty in the classification of plaque components. The CNN had a 2D modified U-net structure6 with 11 layers. The CNN output was grouped with the 4-channel aggregated MR images. The BNN had a Bayesian U-net structure with 10 layers. We randomly selected 80% of our whole dataset as the training set and used the remaining 20% of training data as a testing set after applying the CNN and BNN.
Model test of reproducibility and reliability: First, we assessed the accuracy of the proposed method on the task of segmenting the vessel wall, the carotid plaque, and other regions. Segmentation accuracy was quantified using Dice scores. Second, a reproducibility test7 was conducted to address the concern of outperformance of our model within the limited dataset. The training data was separated into two subsets, each trained separately. The similarity of outputs corresponding to two subsets was measured to show the reproducibility of our model. Finally, we compared the performance of our method to those obtained from two trained readers who were asked to segment and classify the test data using a standard customized software (Image Analysis Suite).
Results
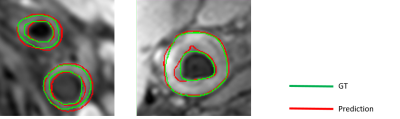
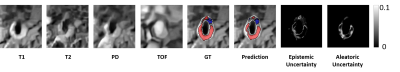
(1) Figure 3 shows two examples of vessel wall segmentation. The Dice coefficients with our approach is 0.84, showing the accuracy of our model in segmenting the vessel wall. The performance of test is given in Table 1.(2) Figure 4 shows an example of carotid plaque segmentation with different tissue types in different colors: LRNC with hemorrhage in red, calcification in blue and fibrous tissues in gray. Aleatoric uncertainty and epistemic uncertainty maps are also shown in Figure 4, showing the ability of our model to estimate uncertainty.
(3) Table 2 shows the results of reproducibility test. The similarity of results from two independent training procedures is as high as 0.85 in Dice coefficient, which illustrates the strong reproducibility of our model.
(4) Table 3 shows the results of the test comparing the performance of our method with trained readers. Our model outperformed two observers on all tissue types.
Discussion and Conclusion
In this study, we established a two-module deep-learning-based approach with modified U-Net and Bayesian U-Net architecture to segment and characterize vessel wall and atherosclerotic plaque. The application of Bayesian U-Net in our model provides a promising way to illustrate and measure the uncertainty of the stochastic segmentation task. The strong reproducibility and reliability of our model has been demonstrated in this study.Acknowledgements
No acknowledgement found.References
1. Dabov K, Foi A, Katkovnik V, et al. Image denoising with block-matching and 3D filtering[C]//Image Processing: Algorithms and Systems, Neural Networks, and Machine Learning. International Society for Optics and Photonics, 2006, 6064: 606414.
2. Reza A M. Realization of the contrast limited adaptive histogram equalization (CLAHE) for real-time image enhancement[J]. Journal of VLSI signal processing systems for signal, image and video technology, 2004, 38(1): 35-44.
3. Wells III W M, Viola P, Atsumi H, et al. Multi-modal volume registration by maximization of mutual information[J]. Medical image analysis, 1996, 1(1): 35-51.
4. Saam, T., et al. "Quantitative evaluation of carotid plaque composition by in vivo MRI." Arteriosclerosis, thrombosis, and vascular biology 25.1 (2005): 234-239.
5. Domingos P. Bayesian averaging of classifiers and the overfitting problem[C]//ICML. 2000, 747: 223-230.
6. Ronneberger, Olaf, Philipp Fischer, and Thomas Brox. "U-net: Convolutional networks for biomedical image segmentation." International Conference on Medical image computing and computer-assisted intervention. Springer, Cham, 2015.
7. Gundersen O E, Kjensmo S. State of the art: Reproducibility in artificial intelligence[C]//Thirty-second AAAI conference on artificial intelligence. 2018.
Figures