4891
Hypertension is Associated with Intracranial Atherosclerotic Plaque Burden and Distribution: a MR Vessel Wall Imaging Study1Radiology, Keck School of Medicine, Los Angeles, CA, United States, 2Radiology, Hospital of the University of Pennsylvania, Philadelphia, PA, United States, 3Neurology, Keck School of Medicine, Los Angeles, CA, United States, 4Radiology, Xuanwu Hospital, Beijing, China, 5Radiology, Beijing Chaoyang Hospital, Beijing, China, 6Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 7Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 8Radiation Oncology, Keck School of Medicine, Los Angeles, CA, United States, 9Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Hypertension is one of the most important modifiable risk factor for atherosclerosis. However, the association between blood pressure and intracranial plaque remains unclear. In this study, we investigated the impact of hypertension on intracranial plaque burden and distribution in 174 patients with recent ischemic stroke. Systolic blood pressure and diastolic blood pressure were independently associated with a higher total plaue burden as well as higher plaque counts at the proximal and junctional locations. These findings suggest hypertension may influence not only the count burden but also the distribution of intracranial plaque development.
Introduction
Hypertension is an important risk factor for atherosclerosis. However, the association between blood pressure and intracranial plaque remains unclear. Our goal was to evaluate the impact of hypertension on intracranial atherosclerotic plaques, especially its burden and distribution, in patients with ischemic stroke using 3D MR vessel wall imaging (MR-VWI).Methods
Adult subjects who underwent 3D MR-VWI within 8 weeks of ischemic stroke onset were retrospectively identified from a prospective database. The exclusion criteria were: (1) image quality was unsatisfactory for analysis; (2) intracranial artery occlusion; (3) patients with endovascular intervention or surgical procedures of intracranial arteries. Patients underwent MR-VWI on a 3.0-Tesla scanner (MAGNETOM Prisma, Verio, Skyra, Siemens, Erlangen, Germany) using a 64-channel head-neck coil (Prisma), 32-channel head coil (Verio), or 20-channel head-neck coil (Skyra). MR-VWI was performed using a recently proposed cerebrospinal fluid-suppressed whole-brain 3D variable-flip-angle turbo spin-echo sequence1, 2. Imaging parameters for MR-VWI were as follows: repetition time, 900ms; echo time, 15 ms; voxel size, isotropic 0.53 or 0.55 mm; field of view, 170×170 mm2 or 170×210 mm2; number of slices, 224 or 240; scan time, approximately 8 minutes. The total number and distribution of plaques were analyzed on MR-VWI images by one blinded neuroradiologist with 5 years of experience in plaque assessment. An atherosclerotic plaque was defined as eccentric wall thickening with or without luminal stenosis3. All plaques identified in the large intracranial arteries, including the intracranial internal carotid arteries (ICA), the anterior cerebral arteries (ACA, A1-A2 segments), middle cerebral arteries (MCA, M1-M3 segments), vertebral arteries (VA, V4 segments), basilar artery (BA), and posterior cerebral arteries (PCA, P1-P2 segments) were analyzed. Proximal plaques included lesions located at the bilateral ICA, A1-ACA, M1-MCA, VA, P1-PCA and BA. The rest were defined as distal plaques. The number of plaques formed at the junction of two arterial segments was recorded to assess the impact of blood pressure on curves and arterial bifurcations. Total plaques are the sum of proximal and distal plaques. Demographic and clinical data were collected from the clinical records. Blood pressure measurements during the first three days of admission were recorded and the average of the three measurements was used in the statistical analysis. Poission or negative binomial model was used depending on data overdispersion which was tested by a dispersion parameter in the negative binomial model. A model-building strategy recommended by Harrell4 was used to identify potential confounders from the demographic data and laboratory measurements. All candidate covariates were entered into a ElesticNet model to select important predictors for plaque count at each segment with predicted residual sum of squares (CVPRESS) as the stopping criteria. The final model will include either systolic blood pressure (SBP) or diastolic blood pressure (DBP) with covariates selected by the previous ElesticNet model. The association was interpreted as a relative risk associated with a 10 mmHg increase of SBP or DBP. Model integrity was examined using Pearson residual pannel. SAS 9.4 was used for all data analysis.Results
A total of 174 patients with ischemic stroke were eligible for the study (Table 1). A median of 7 plaques were identified as total plaque burden. The medians of the proximal, distal and junctional plaques counts were 5, 2 and 2, respectively. After adjusting for potential confounders, every 10 mmHg increase in SBP and DBP were associated with a relative risk (ratio of count) of 1.09 (95%CI 1.04-1.14) and 1.1 (95%CI 1.01-1.18) respectively in the total plaque counts. For proximal plaque counts, the relative risk was 1.1(95%CI 1.05-1.15) and 1.09 (95%CI 1.01-1.18), respectively. No statistically significant association was observed in distal plaque burden. For junctional plaque counts, the relative risk was 1.11 (95%CI 1.04-1.18) and 1.11 (95%CI 1-1.23), respectively (Table 2). Representative cases of the non-hypertension group (Figure 1) and hypertension group (Figure 2) are shown.Discussion
It is widely known that hypertension is an important risk factor for intracranial atherosclerosis. We found that higher SBP and DBP were both independently associated with higher plaque burden, higher counts of proximal but not distal plaques, and higher counts of junctional plaques. Our findings suggest that hypertension not only promotes atheroma formation, but also has a significant impact on the distribution of plaques, especially at the proximal and junctional cerebral artery segments. Different from the carotid arteries, cerebral arteries have many branches that cause a marked drop in blood pressure from large and medium-sized cerebral vessels to cerebral peripheral beds1. Thus, the distal segments appear to be less affected by hypertension. In addition, at bifurcations with curvatures, blood flow tends to be disturbed by nonuniform and irregular distribution of low shear stress2, resulting in lipid accumulation3. Hypertension may contribute to low wall shear stress, which leads to plaque formation at artery junctions.Conclusion
MR-VWI revealed that increases in SBP and DBP were independently associated with a higher burden of total, proximal and junctional plaques. These results suggest that hypertension is an important mechanistic factor for the development of intracranial atherosclerosis. Hypertension may interact with local hemodynamic factors to promote the pathological process of intracranial plaque formation with a predilection for proximal and junctional distributions.Acknowledgements
No acknowledgement found.References
1. Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017;77:1142-1150.
2. Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging 2017;46:751-757.
3. Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534-542.
4. Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag, 2001: Page 63-102.
5. Blanco PJ, Muller LO, Spence JD. Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol 2017;2:108-117.
6. Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 2011;91:327-387.
7. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 2005;85:9-23.
Figures
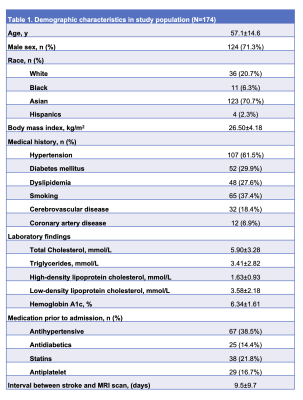
Table 1. Demographic characteristics in study population (N=174).
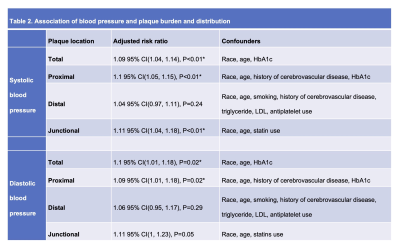
Table 2. Association of blood pressure and plaque burden and distribution.
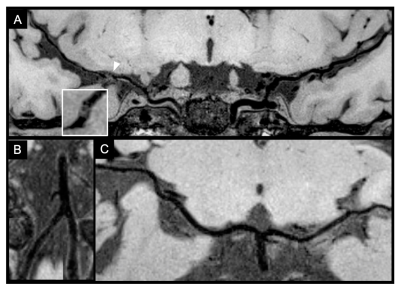
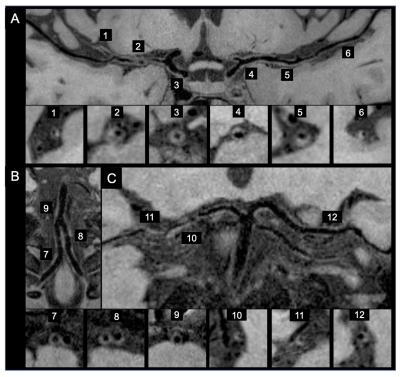
Figure 1. A 49-year-old Asian female patient with an ischemic stroke in the right middle cerebral artery (MCA) territory. She had no history of hypertension and her average blood pressure was 130/80 mmHg. One plaque is shown in the right MCA M1 segment (arrowhead) on the curved multiplanar reconstruction and cross-sectional MR vessel wall imaging (A). No plaque was found in bilateral anterior cerebral arteries (not shown) bilateral vertebral arteries, basilar artery (B) and bilateral posterior cerebral arteries (C) on the curved multiplanar reconstruction images.