4886
Augmented Perfusion Quantified by MRI Depends on Collateral Recruitment in Middle Cerebral Artery Occlusion: A Pilot Study
Mira Liu1, Niloufar Saadat1, Yong Jeong1, Steven Roth2, Marek Niekrasz1, Gregory Christoforidis1, and Timothy Carroll1
1University of Chicago, Chicago, IL, United States, 2University of Illinois, Chicago, IL, United States
1University of Chicago, Chicago, IL, United States, 2University of Illinois, Chicago, IL, United States
Synopsis
We have developed an experimental model and quantitative MRI biomarkers to quantify the effects of therapeutic intervention to enhance collateralization in acute stroke. We performed controlled experiments in a pre-clinical canine model of ischemic stroke and use quantitative cerebral MR perfusion with DSC, intravoxel incoherent motion (IVIM), and neutron capture microsphere deposition, x-ray angiography for collateralization, and MR diffusion imaging for infarction to demonstrate a significant increase in perfusion and decrease in infarct growth in the hyperacute phase of an ischemic stroke.
INTRODUCTION:
During an acute stroke, an infarction core is surrounded by a decreasing penumbra of salvageable ischemic tissue. On average, the persistent perfusion deficit resulting from arterial occlusion kills 1 million neurons per minute. As such there is an ongoing unmet need to reduce the rate at which neurons die. We have an advanced imaging study of flow augmentation on infarct growth and tissue perfusion in a well-controlled pre-clinical (canine) model of stroke (Middle Cerebral Artery Occlusion: MCAO) in a gyrated (human-like pial collateral recruitment) brain[1]. Our goal is to establish the clinical model, quantify pial collateral recruitment, establish a clinically translatable MRI protocol/validation study and show the role that perfusion and diffusion MRI play in quantifying therapeutic response to neuroprotectants. Using quantitative cerebral MR perfusion (i.e., CBF in ml/100g/min) with DSC, intravoxel incoherent motion (IVIM), and neutron capture microsphere deposition [2], x-ray angiography for collateralization, and MR diffusion imaging for infarction, we are able to demonstrate a significant increase in perfusion and decrease in infarct growth in the hyperacute phase of an ischemic stroke.METHODS:
Experimental Model: All MRI scans were performed on a 3T MRI scanner (Achieva, Philips). Perfusion/diffusion was imaged (n=17) at normocapnia (ETCO2 ~ 30 mmHg), induced hypercapnia (ETCO2 ~ 60 mmHg) and after MCAO; seven treatment subjects received a continuous infusion of norepinephrine (0.1 – 1.52mg/kg/min) and hydralazine (20mg). Pial collateral score (PCS) was quantified 30 minutes post occlusion by assessing arteriographic images using a scoring system. DWI/PWI was assessed at 30-minute intervals for 4 hrs post-occlusion. Perfusion was quantified with DSC and IVIM and validated against microsphere values.Gold Standards: Neutrons capture microspheres, DWI and x-ray angiography were deemed reference standards for perfusion, infarct size and pial collateral recruitment, respectively.
MRI Protocol
DSC: Alterations in tissue perfusion was quantified with a previously reported T1-bookend DSC scan[2]. Protocol consisted of 2D EPI Look-Locker inversion recovery (FOV/Matrix = 220 mm/224, slices/thickness = 1/4mm) with variable delay time and DSC perfusion (FOV/Matrix = 220 mm/224, single shot, EPI, fat saturated, slices/thickness =5/6mm slices, TR/TE = 315/40, Flip angle = 75°, 200 time points) with 3 ml iv Gd.
IVIM: IVIM images were acquired with only 10 b-values (0,111, 222, 333, 444, 556, 667, 778, 889, 1000), whole head (slices/thickness= 50/2mm, FOV = 14764mm, total scan time =332s). Perfusion and diffusion images were calculated with 2-step bi-exponential fitting [3].
Microspheres: Neutron captured microsphere CBF was acquired from microspheres injected (4mL of stable isotope labeled 15$$$\mu$$$ microspheres (STERIspheres, BioPal Inc, Medford, MA), that were analyzed and compared to MRI perfusion sequences.
DTI: Infarct volume was determined using Mean Diffusivity (MD) from DTI 1hrs and 4hrs post-MCAO (TR/TE = 2993/83ms, FA = 90°, BW = 1790Hz/pixel, FOV =160 mm, slices/thickness = 50/2mm, b-values = 0, 800 s/$$$mm^2$$$, 32 directions). Standardized infarct volume calculation considered MD voxels below as infarcted and IVIM infarct volume was calculated with the threshold determined iteratively by least absolute deviation regression.
RESULTS:
The effect of flow augmentation depends on the degree of collateralization, with a significantly decrease in infarct growth in subjects with poor collaterals (-35.7%) and an increase in infarct growth in subjects with good collaterals (+9.57%). Similarly, for poorly collateralized subjects (pial collateral score ≤ 8) the treatment group showed an increase in DSC qCBF compared to the control in both hemispheres; contralateral (+56.3%, t = 1.77, p = .08), ipsilateral (+26.2, t=1.06, p = .22). Paradoxically, treatment of subjects with good collaterals (pial collateral score ≥9) showed a decrease in perfusion compared to the control group distal to the occlusion (-35.0%, t=-1.76, p=.08) while maintaining an increase in perfusion on the contralateral side (+99.9%, t = 2.12, p =.03) (Figure 1).Linear regression analysis (Figure 2) showed strong linearity between all measurements of quantitative CBF: IVIM v. DSC ($$$R^2 = .68$$$, slope = .72, intercept = 34.1); IVIM v. microsphere ($$$R^2 = .80$$$, slope = .77, intercept = 60.4). This finding was further supported by excellent agreement shown on concordance correlation analysis (DSC CCC = .79; microsphere CCC =.86). Strong linearity was also found between IVIM and MD infarct volume ($$$R^2 = .76$$$, slope = .82, intercept = 2.0) further supported by Bland-Altman analysis (mean difference = .92, 95% CI [-5.04, 3.21]).
DISCUSSION AND CONCLUSION:
This study showed that treatment of acute stroke with flow augmentation can be quantified with perfusion MRI. The effects of collateralization on therapeutic response through MR perfusion and infarct growth were quantified. Lastly we found that IVIM values correlate strongly with reference standard images of both perfusion and diffusion in acute stroke suggesting further study of IVIM in perfusion diffusion mismatch.Acknowledgements
Research reported in this publication is supported by the NIH Grant R01NS093908 and NSF DGE-1746045References
1. Saadat, N., et al., Influence of simultaneous pressor and vasodilatory agents on the evolution of infarct growth in experimental acute middle cerebral artery occlusion. J Neurointerv Surg, 2020.2. Jeong, Y.I., et al., Absolute quantitative MR perfusion and comparison against stable-isotope microspheres. Magn Reson Med, 2019.
3. Le Bihan, D., et al., Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 1988. 168(2): p. 497-505.
Figures
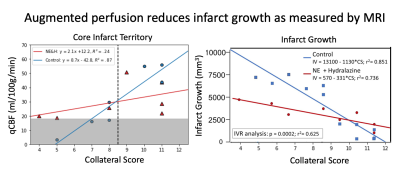
Figure 1. a) Linear regression across collateral score for average DSC qCBF of MCA region ROI for ipsilateral perfusion. A black line divides collateral recruitment score into those determined to be ‘good’ or ‘poor’. b) Predicted and measured infarct growth for treatment and control groups.
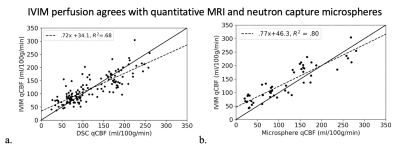
Figure 2. a) Hemispheric correlation of DSC qCBF vs. quantitative IVIM qCBF and b) hemispheric correlation of microsphere qCBF vs. IVIM qCBF across all physiologic states. Dashed lines in linear regression plots represent the line of regression and a solid line of unity is shown for reference. Bland-Altman analysis returned DSC mean difference = -1.3, 95% CI [-71.9, 69.1] and microsphere mean difference = -19.8, 95% CI [-84.0, 44.5].
DOI: https://doi.org/10.58530/2022/4886