4877
Mapping the Age Effect on Cortical Arterial Arrival Time and Perfusion using multi-delay pCASL1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Multi-delay arterial spin labeling (ASL) has made it possible to non-invasively quantify cerebral hemodynamic changes in aging, including cerebral blood flow (CBF) and arterial arrival time (ATT). However, limited numbers of studies exist to focus on the spatial heterogeneity of age effects on both CBF and ATT. In this study, we map the relationships between age, CBF and ATT on the cerebral cortex using multi-delay pseudo-continuous ASL (pCASL) data from the Human Connectome Project in Aging.
Introduction
Multi-delay pCASL provides reliable measures on both CBF and ATT. CBF measures the volume of arterial blood delivered to 100g of brain tissue per minute; ATT describes the time it takes for the labeled blood to travel from the tagging plane to the brain tissue3,4. While the effect of healthy aging on CBF has been heavily studied, ATT is less comprehensively studied, although it also provides important physiologic information on the status of hemodynamic alteration5. Prior research has shown that CBF progressively decreases during aging while ATT is positively associated with age in whole-brain or specific regions-of-interest (ROIs)6,7. Recent studies also indicated that the age-hemodynamic association as revealed by CBF and ATT is region-dependent 8,9, but there has yet to be a study showing variations in ATT-age associations (in conjunction with CBF) across the brain. Thus, the present study aims to study age-related hemodynamics by examining and comparing the magnitude of CBF and ATT changes with age across the cerebral cortex.Methods
Multi-delay pseudo-continuous ASL (pCASL) in the Human Connectome Project-Aging (HCP-A) Lifespan Study helps understand the age-associated hemodynamic changes across the brain1-3. All subjects of this study were participants of the HCP-A Lifespan Study3, which is made of adults without major diagnosed diseases that cause cognitive declines such as stroke and dementia10. Additional manual quality control leads to the exclusion of 147 out of 689 subjects. The final sample consists of 542 subjects aged 36-100, among which, there are 306 females and 236 males.pCASL images were obtained on a Siemens Prisma 3 Tesla scanner using a 2D MB-EPI strategy3. Five post-labeling delays (0.7, 1.2, 1.7, 2.2, 2.7 s) were applied and a tag and control pair is included in each repeat3, which gave a total of 86 frames with an additional 2 frames for the calibration images.
For data processing, TOPUP was used to correct susceptibility-induced distortions, and then OXFORD-ASL was used to quantify CBF and ATT and also to correct for the partial volume effects11,12. Cortical reconstruction was performed with the structural MRI (MPRAGE) data from the same dataset using the software FreeSurfer13. With the same software, CBF and ATT maps were projected onto the cortical surface and then registered to a standard (fsaverage) space.
Voxelwise linear fitting was conducted and the effect size (difference in metric per year) was computed with FreeSurfer’s mri_glmfit14. Clusterwise correction was done for multiple comparisons with the cluster-defining threshold set p<0.05, and alpha=0.05.
Results
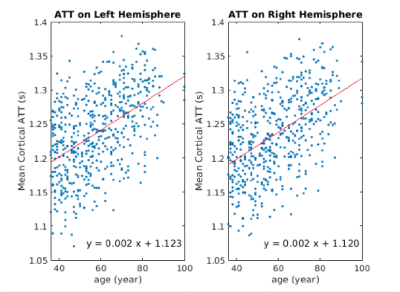
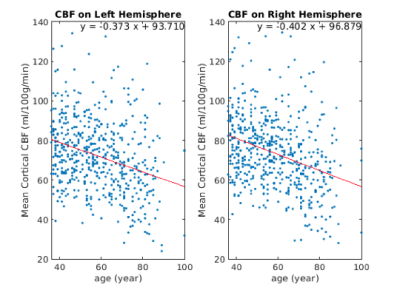
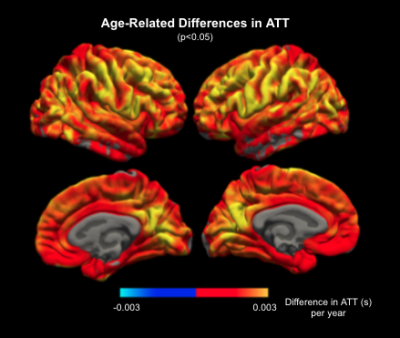
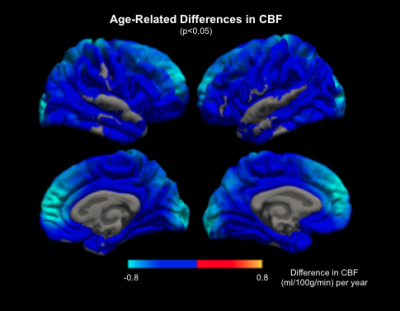
Increasing ATT with age was observed in the cortical grey matter with a mean slope of +0.0002s per year for both left and right hemispheres (Figure 1). CBF is found to be inversely associated with age with the slope of -0.373 ml/100g/min for the left hemisphere and -0.402 ml/100g/min for the right hemisphere (Figure 2).Figure 3 shows the widespread positive association between ATT and age across the cerebral cortex, with the effect size masked by significant clusters. The highest magnitude of age-associated lengthening of ATT was identified in the supramarginal, postcentral, precentral, caudal middle frontal, lingual and precuneus gyrus on both hemispheres. Figure 4 displays negative associations of CBF with age across the cerebral cortex. The peak age effects are distributed on the lateral occipital, postcentral, rostral middle frontal and superior frontal regions of both the right and left cortex. The peak age effects for CBF and ATT do not overlap.
Discussion and Conclusion
In this study, we examined how hemodynamics could gradually change on the cerebral cortex in a typical aging process. An overall positive association of ATT with age and a negative association of CBF with age was described, which is in good agreement with previous ASL studies6,15-17. More importantly, we found that the changes of CBF and ATT with age are not uniform across cortical regions. The lack of spatial overlap between ATT and CBF age effects highlights the necessity of bringing ATT onto the table to be interpreted jointly with CBF. Note that in this work, CBF is quantified using the ATT estimation, thus conventional single-delay ASL results could confound long ATTs with low CBFs. Furthermore, our CBF and ATT estimates are corrected for partial-volume effects, but the pattern of ATT-age associations rather resembles that of age-related cortical thinning (REF). This potential link will be studied in depth in our future work.Acknowledgements
This research has been conducted using the HCP-A data. We thank the CIHR and NSERC for funding support.References
1. Zhang N, Gordon ML, Goldberg TE. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neuroscience & Biobehavioral Reviews. 2017 Jan 1;72:168-75.
2.Eichner C, Jafari‐Khouzani K, Cauley S, Bhat H, Polaskova P, Andronesi OC, Rapalino O, Turner R, Wald LL, Stufflebeam S, Setsompop K. Slice accelerated gradient‐echo spin‐echo dynamic susceptibility contrast imaging with blipped CAIPI for increased slice coverage. Magnetic resonance in medicine. 2014 Sep;72(3):770-8.
3. Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, Coalson TS. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage. 2018 Dec 1;183:972-84.
4. Buxton RB. Quantifying CBF with arterial spin labeling. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005 Dec;22(6):723-6.
5. Derdeyn CP, Grubb RL, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999 Jul 1;53(2):251-.
6. Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, Schuff N. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magnetic resonance in medicine. 2012 Sep;68(3):912-22.
7. Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral cortex. 2011 Jun 1;21(6):1426-34.
8. Pavilla A, Arrigo A, Colombani S, Mejdoubi M. Absolute and regional cerebral perfusion assessment feasibility in head-down position with arterial spin-labeling magnetic resonance. A preliminary report on healthy subjects. Journal of Neuroradiology. 2016 Dec 1;43(6):392-7.
9. Juttukonda MR, Li B, Almaktoum R, Stephens KA, Yochim KM, Yacoub E, Buckner RL, Salat DH. Characterizing cerebral hemodynamics across the adult lifespan with arterial spin labeling MRI data from the Human Connectome Project-Aging. NeuroImage. 2021 Apr 15;230:117807.
10. Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, Fischl B. The lifespan human connectome project in aging: an overview. Neuroimage. 2019 Jan 15;185:335-48.
11. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003 Oct 1;20(2):870-88.
12. Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian inference for a nonlinear forward model. IEEE Transactions on Signal Processing. 2008 Sep 16;57(1):223-36.
13. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000 Sep 26;97(20):11050-5.
14. Mutsaerts HJ, Petr J, Václavů L, Van Dalen JW, Robertson AD, Caan MW, Masellis M, Nederveen AJ, Richard E, MacIntosh BJ. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. Journal of Cerebral Blood Flow & Metabolism. 2017 Sep;37(9):3184-92.
15. Asllani I, Habeck C, Borogovac A, Brown TR, Brickman AM, Stern Y. Separating function from structure in perfusion imaging of the aging brain. Human brain mapping. 2009 Sep 15;30(9):2927-35.
16. Ambarki K, Wåhlin A, Zarrinkoob L, Wirestam R, Petr J, Malm J, Eklund A. Accuracy of parenchymal cerebral blood flow measurements using pseudocontinuous arterial spin-labeling in healthy volunteers. American Journal of Neuroradiology. 2015 Oct 1;36(10):1816-21.
Figures