4876
Highly Accelerated Myocardial Perfusion Using Physics-guided Deep Learning With Structure-encoded Coil Maps1Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Department of Imaging Physics, Delft University of Technology, Delft, Netherlands
Synopsis
Myocardial perfusion cardiac MRI is widely used to functionally assess coronary artery disease. Although numerous acceleration techniques are used, improved spatio-temporal resolutions and coverage are desirable. Deep learning (DL) reconstruction has shown improvement over conventional reconstruction techniques at higher accelerations. Yet, SNR/contrast changes across perfusion dynamics hinder their generalization performance. In this work, we propose a multi-coil encoding operator that uses coil maps encoding structural information for physics-guided DL. This provides a uniform contrast at the network output across dynamics, leading to improved image quality compared to physics-guided DL with ESPIRiT coil maps, as well as conventional acceleration methods.
INTRODUCTION
Myocardial first-pass perfusion cardiac MRI (CMR) enables assessment of the functional significance of coronary artery disease. Perfusion CMR is acquired using snap-shot imaging during the passage of a contrast agent, which requires trade-offs between resolution and coverage1,2. Numerous techniques based on parallel or simultaneous multi-slice (SMS) imaging, and compressed sensing have been proposed to tackle this challenge3,4. Recently, physics-guided deep learning (PG-DL) has shown exceptional reconstructions at highly accelerated imaging, where conventional methods suffer from residual aliasing and noise5,6. However, PG-DL faces several challenges on its own. PG-DL struggles to generalize when SNRs/contrasts change between training and testing7. A possible solution is to regularize over spatio-temporal (2D+t) domain, but this is prone the temporal blurring, and it is hard to procure large high-quality databases due to changes in contrast dynamics and breathing patterns among subjects. In this work, we tackle these challenges by using structure-encoded maps that capture SNR/contrast changes across dynamics leading to improved generalizability for PG-DL, while reconstructing each dynamic individually. The proposed approach was applied to highly-accelerated myocardial perfusion using self-supervised PG-DL with structure-encoded maps. The proposed method improves on parallel imaging and conventional PG-DL reconstructions.METHODS
Theory: The inverse problem of MRI reconstruction is given as:$$\hat{x}_{reg} = \arg \min_{\mathbf{x}} \left\| \mathbf{y}-\mathbf{E}\mathbf{x} \right\|_{2}^{2} + {\cal{R}}(\mathbf{x}), \quad\quad (1)$$
where $$$\mathbf{x}$$$ is the image of interest, $$$\mathbf{y}$$$ is the acquired multi-coil k-space, $$$\mathbf{E}$$$ is the multi-coil encoding operator and $$$\cal{R}(\cdot)$$$ is the regularizer. The encoding operator is formed as $$$\mathbf{E}=[\mathbf{F}_{\Omega}\mathbf{C}_{1};\cdots;\mathbf{F}_{\Omega}\mathbf{C}_{K}]$$$ where $$$\mathbf{F}_{\Omega}$$$ is a partial Fourier operator sampling indices $$$\Omega$$$, and $$$\mathbf{C}_{K}$$$ is the kth coil map. Conventionally, these maps are generated using ESPIRiT8, which leads to normalized maps, i.e. $$$\sum_k \mathbf{C}_{k}^{*}\mathbf{C}_{k}={\bf I}$$$, and these do not inherently capture contrast variations across different dynamics.
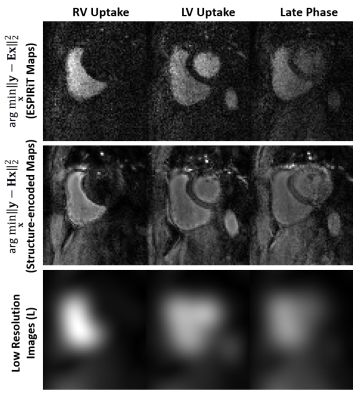
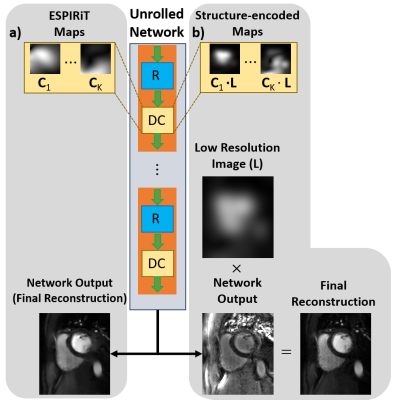
As an alternative, we propose to encode contrast/SNR in the coil-maps for PG-DL. Let $$$\mathbf{L}$$$ be an image that contains contrast information for a given dynamic, such as a low-resolution image. We define the structure-encoded coil maps as $$$\mathbf{L}_{K} = \mathbf{C}_{K}\cdot\mathbf{L}$$$. This leads to a reformulated multi-coil operator $$$ \mathbf{H}=\mathbf{E}\mathbf{L}$$$ with the corresponding inverse problem:
$$ \hat{x}_{struct} = \arg \min_{\mathbf{x}} \left\| \mathbf{y}-\mathbf{H}\mathbf{x} \right\|_{2}^{2} + {\cal{R}}(\mathbf{x}). \quad\quad (2)$$
It is easy to show that for the unregularized case9,
$$\hat{x}_{reg} = (\mathbf{E}^{*}\mathbf{E})^{-1}\mathbf{E}^{*}\mathbf{y} = \mathbf{L}\cdot\hat{x}_{struct}. \quad\quad (3)$$
The main advantage of the proposed formulation is that the solutions to Eq. 2 have uniform contrast across different dynamics, facilitating generalizability for PG-DL (Fig. 1).
Imaging Experiments: Free-breathing first-pass perfusion CMR was acquired at 3T on 8 subjects with 12-fold acceleration (SMS=3, uniform Cartesian in-plane R=4). A saturation-prepared GRE sequence with slab-selective outer volume suppression10 was used with resolution=1.7×1.7mm2, FOV=360×360mm2, temporal resolution=110ms, slice-thickness=8mm. A separate calibration scan was performed with resolution=1.7×5.6mm2, FOV= 360×360mm2.
Implementation Details: First, ESPIRiT8 was used to generate conventional coil sensitivity maps ($$$\mathbf{C}_K$$$) using the central 24×24 region of the calibration scan. Then, split slice-GRAPPA11 was used to generate each dynamic. Note that this step was necessary due to lack of individual k-spaces of the slices due to SMS encoding and would not be needed for single-slice or single-volume imaging. Subsequently, low-resolution images ($$$\mathbf{L}$$$) were estimated using central 24×24 region followed by a ringing filter of each reconstructed dynamic. Finally, $$$\mathbf{C}_K$$$’s from first step were multiplied by $$$\mathbf{L}$$$ to generate the structure-encoded coil maps.
Physics-guided deep learning (PG-DL) training was performed on 4 subjects using self-supervised learning via data undersampling (SSDU), which does not require fully-sampled data12. Algorithm unrolling based on variable splitting for 10 iterations was used12. Training was performed over 360 SMS k-spaces using Adam optimizer, learning rate=$$$3\cdot10^{-4}$$$, 100 epochs and normalized $$$\ell_1-\ell_2$$$ loss. Two trainings were performed with same settings, one with conventional ESPIRiT maps and one with proposed structure-encoded coil maps (Fig. 2). Note the structure-encoded network outputs were multiplied with respective low-resolution images ($$$\mathbf{L}$$$) for the final reconstruction, as in Eq. 3.
RESULTS
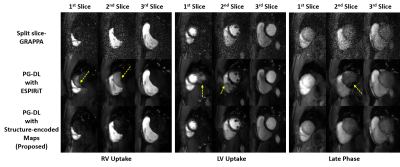
Fig. 3 shows reconstructed slices from an SMS slice group across different dynamics. Split slice-GRAPPA reconstruction reduces aliasing, but has high noise amplification. PG-DL with conventional encoding operator using ESPIRiT maps reduces noise, but has visible aliasing (yellow arrows). Proposed approach suppresses both aliasing noise amplification.Fig. 4 depicts reconstructions of the whole heart with 9 slices from 3 SMS groups. Proposed approach shows improved image quality compared to PG-DL with ESPIRiT and split slice-GRAPPA, which suffer from aliasing and noise amplification respectively.
DISCUSSION AND CONCLUSIONS
In this study, we proposed a contrast-aware encoding operator for PG-DL using structure-encoded coil-maps that capture SNR/contrast information across an image series. The proposed structure-encoded maps were used for PG-DL reconstruction of highly accelerated myocardial perfusion, and showed improved image quality compared to conventional ESPIRiT map based encoding operator. ESPIRiT maps do not exhibit variations across dynamics whereas in structure-encoded maps, SNR information is captured in the maps leading a more uniform contrast across dynamics at the network output. This in turn makes it easier for the deep learning reconstruction to generalize for perfusion CMR, when SNR levels fluctuate with inherent physiological processes.Acknowledgements
Funding: Grant support: NIH R01HL153146, NIH P41EB027061, NIH R21EB028369, NSFCCF-1651825. NWO STU.019.024, 4TU Federation and AHA Predoctoral Fellowship.References
[1] R. Franks, C. Amedeo and P. Sven, "Clinical application of dynamic contrast enhanced perfusion imaging by cardiovascular magnetic resonance." Frontiers in Cardiovascular Medicine (2021).
[2] M. Jerosch-Herold, “Techniques for MR myocardial perfusion imaging,” in J Cardiovasc Magn Reson, pp.99–112. Springer, 2019
[3] Y. Yang, et al. "Whole‐heart spiral simultaneous multi‐slice first‐pass myocardial perfusion imaging." Magnetic Resonance in Medicine 81.2 (2019): 852-862.
[4] M.S. Nazir, et al. "Simultaneous multi slice (SMS) balanced steady state free precession first-pass myocardial perfusion cardiovascular magnetic resonance with iterative reconstruction at 1.5 T." J Cardiovasc Magn Reason, 20.1 (2018): 1-11.
[5] K. Hammernik, T. Klatzer, et al., “Learning a variational network for reconstruction of accelerated MRI data,” Magn Reson Med, vol. 79, pp. 3055–3071, 2018.
[6] J. Schlemper, J. Caballero, J. V. Hajnal, A. N. Price, and D. Rueckert, “A deep cascade of convolutional neural networks for dynamic MR image reconstruction,” IEEE Trans Med Imaging, vol. 37, no. 2, pp. 491–503, 2017.
[7] F. Knoll, K. Hammernik, et al., “Assessment of the generalization of learned image reconstruction and the potential for transfer learning,” Magn Reson Med, vol. 81, no. 1, pp. 116 128, 2019.
[8] M. Uecker, P. Lai, et al., “ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA,” Magn Reson Med, vol. 71, no. 3, pp. 990–1001, 2014.
[9] D. K. Sodickson and C. A. McKenzie, “A generalized approach to parallel magnetic resonance imaging,” Med Phys, vol. 28, no. 8, pp. 1629–1643, 2001.
[10] S. Weingärtner, S. Moeller, and M. Akçakaya, “Feasibility of ultra-high simultaneous multi-slice and in-plane accelerations for cardiac MRI using outer volume suppression and leakage-blocking reconstruction,” in Proc ISMRM, 2018
[11] S. F. Cauley, J. R. Polimeni, H. Bhat, L. L. Wald, and K. Setsompop, “Interslice leakage artifact reduction technique for simultaneous multislice acquisitions,” Magn Reson Med, vol. 72, no. 1, pp. 93–102, 2014.
[12] B. Yaman, S. A. H. Hosseini, et al., “Self-supervised learning of physics-guided reconstruction neural networks without fully sampled reference data,” Magn Reason Med, vol. 84, no. 6, pp. 3172–3191, 2020.
Figures