4874
Potential regulation of cerebral blood flow by the basal forebrain1Computer Science, State University of New York at Binghamton, Binghamton, NY, United States, 2Department of Psychology, Cornell University, Ithaca, NY, United States
Synopsis
The basal forebrain (BF) has a known regulatory role in the cholinergic vasodilatory system, increasing cerebral blood flow (CBF) in the cerebral cortex using animal studies. However, parallel human studies have never been performed. Thirty-three young adults and 27 older adults were tested for the aged-related changes in arterial transit time (ATT) and CBF, which were measured using arterial spin labeling (ASL). We observed the age-related CBF and ATT changes in the basal forebrain and other brain regions, which provide a foundation for investigating the regulatory role of the basal forebrain on the cholinergic vasodilatory system.
Introduction
The basal forebrain (BF), containing large neurons with similar cells and producing cholinergic projections to different brain regions1, has received particular attention due to its atrophy in Alzheimer’s disease2, 3. Animal studies have shown that chemical and electric stimulation in cholinergic projections from basal forebrain neurons causes vasodilation in the cerebral cortex4-7, which increases cerebral blood flow (CBF) in the frontal, parietal, and occipital regions. However, due to the invasiveness of such stimulation, this cholinergic vasodilator system has not been demonstrated in humans. Here, we investigated the potential role of the basal forebrain on CBF and age-related CBF changes in its regulation. The CBF-related measures were performed using the noninvasive arterial spin labeling (ASL) technique.Methods
The study was conducted at the Cornell University MR Facility using a GE 3T MR 750. All healthy subjects (33 young adults: 30.82 .56 years, 23 females; 27 older adults: 68.63 4.84 years, 15 females) received T1-weighted MPRAGE images, a low-resolution arterial transit time (ATT) acquisition8 with five post-labeling delays of 0.7 s, 1.3 s, 1.9 s, 2.5 s, 3.0 s, and a dynamic pseudo-continuous arterial spin labeling (PCASL) sequence with 30 three-dimensional ASL image volumes9.ATT maps were derived voxel-by-voxel by applying a noniterative algorithm to the ASL data with five post-labeling delays8. CBF maps were calculated using a standard kinetic model without ATT correction10, 11. The ATT and CBF maps were normalized to a standard MNI template using the T1-weighted images as an intermediate, and smoothed with an 8×8×8 mm3 Gaussian kernel. Contrast maps between the younger and older adults were generated using SPM12 via two-sampled t tests with gender as a covariate. The statistical maps were thresholded using a voxel-level p value of 0.001. A cluster-level p value of 0.05 was used to correct for multiple comparisons.
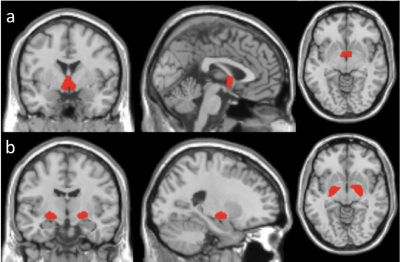
The BF contains different cholinergic (Ch) nuclei. Probability maps of BF Ch1, Ch2, Ch3, and Ch4 were obtained12. BF Ch1, Ch2, Ch3 were combined into one region (BF123) because they are located in connected and adjacent regions, while BF Ch4 was used as a separate region (BF4). The spatial locations of BF123 and BF4 are shown in Fig. 1. The regional ATT and CBF values in the BF123 and BF4 regions were calculated as the average value of the ATT and CBF maps over the corresponding regions. The regional ATT and CBF values in the BF123 and BF4 regions were compared between the younger and older adults using MATLAB via two-sampled t tests with gender as a covariate.
Results & Discussion
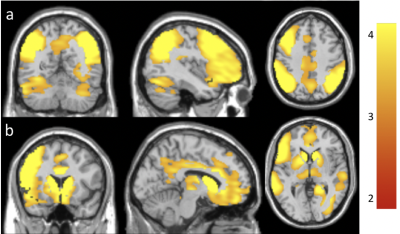
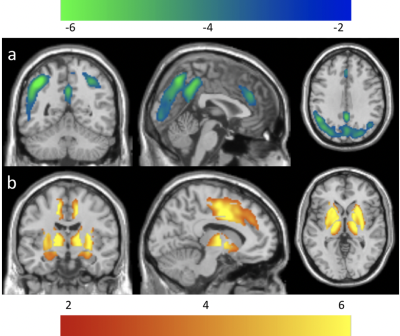
ATT values in the older adults were significantly longer in the frontal, parietal, temporal, and occipital regions (Fig. 2a), and subcortical areas (Fig. 2b) than those in the younger adults, which is consistent with our earlier results13. ATT values in the older adults were also significantly longer in the BF123 (p = 0.0017) and BF4 (p = 0.0031) regions than those in the young adults.CBF values in the older adults were significantly reduced in the prefrontal/anterior cingulate, insular, parietal, and occipital regions (Fig. 3a) but significantly increased in the subcortical areas (Fig. 3b). The regions with reduced CBF in elderly are in good agreement with previous PET studies14. The increased CBF values in the subcortical regions of the elderly agrees well with some literature15,16 but there are inconsistent findings in past studies. CBF values in older adults were significantly increased in the BF123 (p = 4.49e-4) and BF4 (p = 1.57e-6) regions compared to those in the younger adults. The increased CBF in the basal forebrain and decreased CBF in the neocortex may be explained by the age-related reduced acetylcholine (ACh) receptor activity despite the increased Ach release from the basal forebrain. Age-related CBF decreases are consistent with age-related decline of the intracerebral cholinergic vasodilator system (age-related decline of nicotinic ACh receptor activity) found in rats17.
Conclusion
Using human subjects, we observed the age-related CBF and ATT changes in the basal forebrain and other cortical regions, which provide a foundation for investigating the regulatory role of the basal forebrain on the cholinergic vasodilatory system.Acknowledgements
No acknowledgement found.References
1. Mesulam, M.M. and C. Geula, Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol, 1988. 275(2): p. 216-40.
2. Perry, R.H., et al., The substantia innominata and adjacent regions in the human brain: histochemical and biochemical observations. J Anat, 1984. 138 ( Pt 4): p. 713-32.
3. Price, D., P. Whitehouse, and R. Struble, Cellular pathology in Alzheimer's and Parkinson's diseases. Trends in Neurosciences, 1986. 9: p. 29-33.
4. Hotta, H., et al., Involvement of the basal nucleus of Meynert on regional cerebral cortical vasodilation associated with masticatory muscle activity in rats. J Cereb Blood Flow Metab, 2020. 40(12): p. 2416-2428.
5. Uchida, S. and F. Kagitani, Effect of basal forebrain stimulation on extracellular acetylcholine release and blood flow in the olfactory bulb. J Physiol Sci, 2018. 68(4): p. 415-423.
6. Hotta, H., et al., Control of cerebral cortical blood flow by stimulation of basal forebrain cholinergic areas in mice. J Physiol Sci, 2011. 61(3): p. 201-9.
7. Takata, N., et al., Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One, 2013. 8(6): p. e66525.
8. Dai, W., et al., Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012. 67(5): p. 1252-65.
9. Dai, W., et al., Quantifying fluctuations of resting state networks using arterial spin labeling perfusion MRI. J Cereb Blood Flow Metab, 2016. 36(3): p. 463-73.
10. Alsop, D.C. and J.A. Detre, Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of Human cerebral blood flow. J Cereb Blood Flow Metab, 1996. 16: p. 1236-49.
11. Buxton, R.B., et al., A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med, 1998. 40: p. 383-96.
12. Zaborszky, L., et al., Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage, 2008. 42(3): p. 1127-41.
13. Dai, W., et al., Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. J Magn Reson Imaging, 2017. 45(2): p. 472-481.
14. Chen, J.J., H.D. Rosas, and D.H. Salat, Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage, 2011. 55(2): p. 468-78.
15. Lee, C., et al., Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: implications for imaging of neurodegenerative disease. J Neuroimaging, 2009. 19(4): p. 344-52.
16. Pagani, M., et al., Regional cerebral blood flow as assessed by principal component analysis and (99m)Tc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging, 2002. 29(1): p. 67-75.
17. Sato, A., Y. Sato, and S. Uchida, Regulation of cerebral cortical blood flow by the basal forebrain cholinergic fibers and aging. Auton Neurosci, 2002. 96(1): p. 13-9.
Figures