4859
AI-based Fully Automated 4D Flow Image Reconstruction and Post-Processing Pipeline in under 10 minutes.1Biomedical Engineering, Northwestern University, Chicago, IL, United States, 2Cardiac Surgery, Northwestern University, Chicago, IL, United States, 3Radiology, Northwestern University, Evanston, IL, United States
Synopsis
4D flow MRI provides comprehensive assessment of cardiovascular hemodynamics. However, the current clinical usage of standard 4D flow MRI is hindered by long scan times and extensive, time-consuming post-processing such as eddy current corrections, noise masking, and 3D vessel segmentation. We seek to address this by developing a fully automated image reconstruction and post-processing pipeline for highly-accelerated aortic 4D flow MRI (R=5.7-10.2). The fully automated pipeline was shown to provide good-to-excellent agreement in quantitative and hemodynamic measures to conventional 4D flow MRI (GRAPPA, R=2) with manual post-processing. Additionally, the pipeline only requires <10 minutes compared to 20 minutes manually.
Purpose
4D flow MRI provides comprehensive assessment of cardiovascular hemodynamics. However, the current clinical usage of standard 4D flow MRI is hindered by long scan times and extensive, time-consuming post-processing such as eddy current corrections, noise masking, and 3D vessel segmentation. Recent studies have demonstrated reduced 4D flow scan times through sparse k-space sampling and Compressed Sensing (CS)[1,2]. However, iterative CS reconstruction requires lengthy reconstruction times and have shown significant underestimation in hemodynamics (flow, peak velocity) compared to conventional 4D flow MRI, up to 15-25% for peak systolic velocities[1, 2]. Alternatively, convolutional neural networks (CNN) have shown to be highly effective in improving image reconstruction of undersampled k-space data[3-4]. We seek to expand on these developments by implementing a fully automated image reconstruction and post-processing pipeline for highly-accelerated aortic 4D flow MRI. We utilized a dedicated reconstruction-analysis pipeline that included the execution of five CNNs for image reconstruction and subsequent processing in <10 minutes. Our goal was to systematically investigate the pipeline’s performance for different 4D flow acceleration factors (R=5.7-10.2) compared to conventional 4D flow MRI reconstruction and flow analysis.Methods
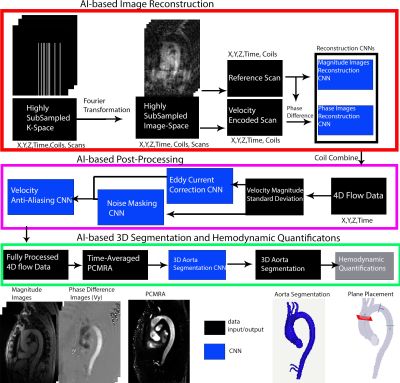
This IRB approved study included 18 prospectively enrolled patients (58 ± 15 y old; 14 M) with aortic disease. Each subject underwent four aortic 4D flow MRI scans: conventional 4D flow MRI with standard parallel imaging (GRAPPA, R=2) and CS accelerated scans with 1) R=5.7, (2) R=7.7, (3) R=10.2. Data acquisition was based on a previously described, variable‐density, Cartesian spiral phyllotaxis sampling pattern[5]. All scans were acquired back-to-back on a 1.5T MRI system (Area, Siemens) with identical 3D coverage, spatial resolution (2.4-4.2 mm3), and velocity encoding. The automated pipeline was constructed to include (1) AI-based reconstruction of 4D flow data, (2)CNN for eddy current correction, (3) CNN to mask noisy voxel in the lungs, (4) CNN-based velocity anti-aliasing, (5)AI-based aortic 3D segmentation(Figure 1). All five CNNs consist of a hybrid 3D DenseNet/U-Net, as previously described[6]. After Fourier transform, complex image-space datasets were used as inputs for the reconstruction pipeline. Subsequently, the standard deviation of the velocity magnitude was used as the input for both the eddy current correction (through the detection of static tissue in the data) and noise masking (removing noisy voxels) CNNs. The velocity antialiasing CNN was applied to correct any phase wrapped voxels in the data. A time-average phase-contrast MRA (PCMRA) was then generated and used as input for the 3D segmentation CNN to generate a 3D aortic segmentation. The pipeline was run on a workstation with i7-8700k and GTX-1080 Ti.To assess the performance of the reconstruction-analysis pipeline, the resulting 4D flow MRI data were compared to conventional 4D flow MRI with manual postprocessing. Manual postprocessing involved user selection of threshold values to determine the static tissue (for eddy current corrections) and noise masking, manually segmenting a 3D aorta from the PCMRA, and the use of a conventional antialiasing algorithm, which detects phase wrapped voxels based on the temporal phase-jumps in the data.
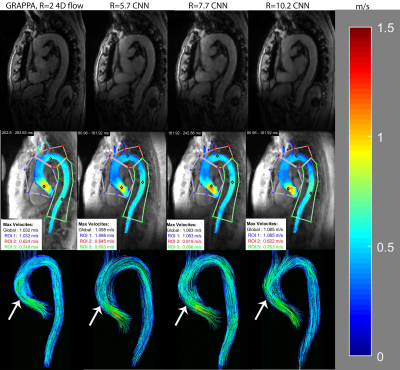
For magnitude images comparison (CNN vs conventional), SSIM values were calculated. Dice scores were calculated to assess the 3D aorta segmentation quality between CNN based and manual analysis. Further, the velocity antialiasing CNN was compared to a conventional velocity antialiasing algorithm based on number of detected wrapped voxels. Hemodynamic analysis involved 3D flow visualization and peak velocity maximum intensity projections, MIPs(Figure 2), as well as quantification of peak velocity, net flow, and peak flow for the ascending aorta(AAo), aortic arch, and descending aorta(DAo) through 2D plane analysis. A t-test or Wilcoxon ranksum test, depending on normality, was used to determine significant differences between AI pipeline and manual analysis.
Results
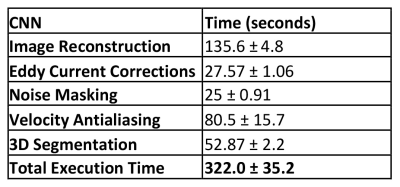
The average time for the entire automated reconstruction and post-processing pipeline was 322.0 ± 35.2 seconds, compared to ~20-30 minutes for CS-reconstruction and manual data analysis (Table 1). CNN reconstructed magnitude images showed good agreement with conventional 4D flow, as documented by median SSIM values: R=5.7, 0.88[0.86-0.89]; R=7.7, 0.87[0.85-0.87]; R=10.2, 0.87[0.86-0.87].For the 3D aortic segmentation, the pipeline had median Dice scores of: R=5.7, 0.90[0.87-0.91]; R=7.7, 0.89[0.86-0.91]; R=10.2, 0.88[0.85-0.89]. The median number of detected wrapped voxels by the velocity antialiasing CNN was: R=5.7, 3378[2084-5362]; R=7.7, 4169[1916-8061]; R=10.2, 3760[2573-4995]. The conventional antialiasing algorithm detected: R=5.7, 4136[1600-6266]; R=7.7, 3681[1532-6774]; R=10.2, 3564[2201-5734]. No significant differences were found between the CNN and the conventional algorithm.
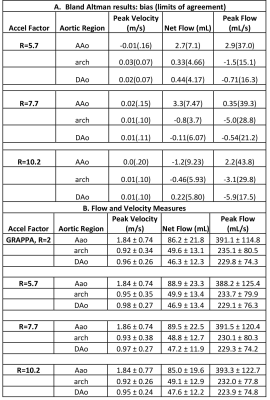
Figure 2 provides examples of magnitude images, MIPs, and 3D streamlines for conventional 4D flow compared to the CNN-reconstructed and processed data for R=5.7, 7.7, and 10.2. The CNNs retained similar velocity profiles and flow patterns as the conventional 4D flow. Table 2 summarizes results of the Bland-Altman comparisons between the conventional GRAPPA 4D flow with manual postprocessing and the automated pipeline as well as the hemodynamic measures across the entire cohort. The fully automated pipeline showed good-to-excellent agreement with conventional 4D flow data with manual postprocessing across all quantifications and acceleration factors, with limits of agreements showing the CNNs had 6.4-12.3% difference from conventional 4D flow reference values.
Discussion
The fully automated pipeline showed good-to-excellent agreement with conventional 4D flow MRI, while requiring <10 minutes. Future direction of this study will utilize the automated pipeline on data with higher acceleration factors and additional hemodynamic metrics.Acknowledgements
No acknowledgement found.References
1. Pathrose, A., et al., Highly accelerated aortic 4D flow MRI using compressed sensing: Performance at different acceleration factors in patients with aortic disease. Magn Reson Med, 2021. 85(4): p. 2174-2187.
2. Ma, L.E., et al., Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k-space reordering, and inline reconstruction. Magn Reson Med, 2019. 81(6): p. 3675-3690.
3. Haji-Valizadeh, H., et al., Highly accelerated free-breathing real-time phase contrast cardiovascular MRI via complex-difference deep learning. Magn Reson Med, 2021. 86(2): p. 804-819.
4. Vishnevskiy, V., J. Walheim, and S. Kozerke, Deep Variational Network for Rapid 4D Flow MRI Reconstruction. Nature Machine Intelligence, 2020. 2.
5. Uecker, M., et al., ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med, 2014. 71(3): p. 990-1001.
6. Berhane, H., et al., Fully automated 3D aortic segmentation of 4D flow MRI for hemodynamic analysis using deep learning. Magn Reson Med, 2020. 84(4): p. 2204-2218.
Figures