4858
EPI with Parallel Imaging (eπ2) self-calibrates the image distortion due to B0 field inhomogeneity1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Correcting image distortions in echo-planar-imaging (EPI) due to B0 field inhomogeneity usually requires additional acquisitions, such as a B0 field map or blip-up-blip-down image pair. Interestingly, susceptibility distortions in EPI with parallel imaging can be corrected by leveraging the mismatch in phase-encoding polarity and/or effective echo-spacing between the auto-calibration-signal and under-sampled data, obviating the need for additional image acquisitions. Here we demonstrate the proposed pipeline of susceptibility distortion correction in in vivo diffusion MRI data of a healthy subject, and the results and estimated field values are comparable with the conventional method.
Introduction
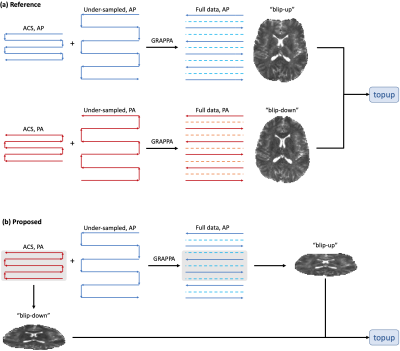
The B0 field inhomogeneity leads to image distortions in echo-planar-imaging (EPI) [1], which can be problematic for the analysis and registration of EPI-based MRI methods, such as diffusion and functional MRI, to non-EPI-based MR images. Conventionally, additional images are required to correct EPI distortions, such as a B0 field map or blip-up-blip-down image pair (Fig. 1a) [2]. Interestingly, in the EPI with parallel imaging (PI), the auto-calibration-signal (ACS) does not need to match the echo-spacing and phase-encoding (PE) direction of the under-sampled data [3]. This flexibility in ACS acquisition allows for distortion mismatch between ACS and under-sampled data. Here, we toggle the PE direction of ACS and under-sampled data, and leverage the distortion mismatch to estimate the B0 field map and correct the susceptibility distortion, thereby obviating the need to acquire additional images (Fig. 1b). As a proof of principle, the proposed pipeline is demonstrated in in vivo diffusion MRI data of a healthy subject, and the results are compared with conventional methods (Fig. 1a) [2].Methods
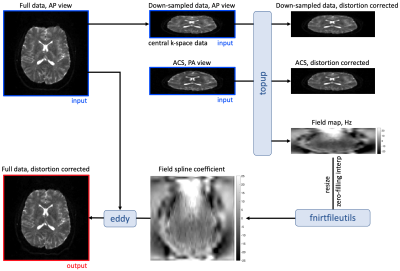
PipelineThe pipeline is illustrated in Figs. 1-2. In the proposed EPI with PI sequence, dubbed as E(PI)2 or eπ2, the ACS and under-sampled data are acquired with opposite PE polarities. Though the ACS and under-sampled data are not matched in image distortions, we can still use the ACS data to train GRAPPA weights and reconstruct the full k-space data [3], whose PE polarity are still the same as that of the under-sampled data. This is because GRAPPA kernels are slowly varying functions of space. The central k-space of the PI-reconstructed full data provides a "blip-up" image for distortion correction. Due to the opposite PE polarity, the ACS data yields another "blip-down" image for correction. This blip-up-blip-down image pair has matched voxel sizes with low resolution in the PE direction, but the image pair is mismatched in the PE direction (and/or echo-spacing). Due to its low resolution in PE, it is necessary to correct the Gibbs-ringing in the blip-up-blip-down image pair by using local subvoxel-shifts [4] before the field map estimation.
We then input the image pair into FSL topup [2] and estimate the B0 field map, re-size and translate the field map into the spline coefficients using FSL fnirtfileutils [5], and correct the susceptibility distortion by using the spline coefficients and FSL eddy [6].
The pipeline will be available on https://github.com/leehhtw.
In vivo MRI
Whole brain diffusion MRI images were acquired in a healthy volunteer on a 3T Siemens Prisma scanner using a 20-channel head coil. Diffusion-weighted pulsed-gradient spin-echo EPI images were acquired with AP phase-encoding, including 4 b=0 images (non-DWIs) and 84 diffusion-weighted-images (DWIs) of b-values = [0.25, 1, 2] ms/µm2 in [4, 20, 60] gradient directions. An additional b=0 image with PA phase-encoding was acquired. To compare our pipeline with the conventional approach, we re-combined the data as follows:
Reference: Two b=0 images in the AP and PA views, respectively, were used to estimate the field map using FSL topup (Fig. 1a). The data of b=0 images for the field map estimation consisted of two ACS data in AP and PA views and two under-sampled data in AP and PA views, respectively. This approach has been widely used in many publicly available databases [7-9].
Proposed: Only one b=0 image was used to estimate the field map based on the pipeline in the previous section (Fig2. 1b-2). The data of b=0 image for the field map estimation consisted of one ACS data in the PA view and one under-sampled data in the AP view.
Other sequence parameters: Voxel size=1.7×1.7×3 mm3. TR/TE=3700/96 ms. GRAPPA with acceleration factor 2, multiband with acceleration factor 2. Maps of mean diffusivity (MD), fractional anisotropy (FA), and mean kurtosis (MK) were calculated [10-11].
Results
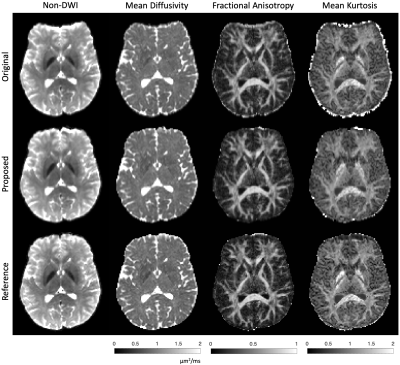
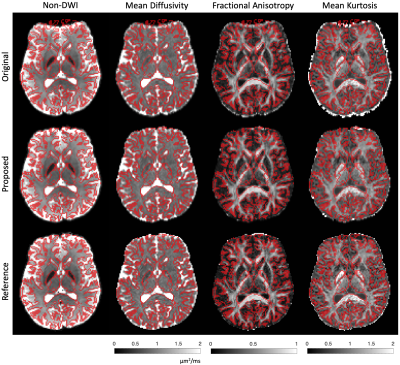
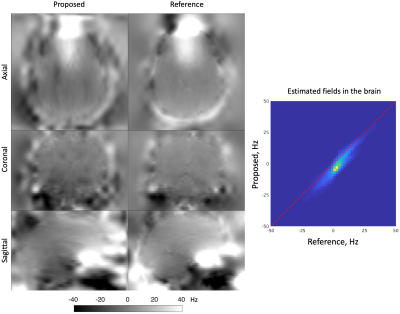
The proposed pipeline successfully corrects susceptibility distortion without the need for additional b=0 image acquisition in the opposite PE polarity (Figs. 3-4). Furthermore, the estimated field map of the proposed pipeline coincides with that of the reference, indicated by the high correlation in field values of the proposed pipeline and reference method (Fig. 5).Discussion and Conclusions
Susceptibility distortions in EPI with PI can be corrected by leveraging the mismatch in PE polarity and/or effective echo-spacing between ACS and under-sampled data, obviating the need for additional acquisitions. Further, the eddy current distortion in DWIs can be corrected by combining the results of proposed pipeline and FSL eddy.The ACS in PI may consist of central k-space data, leading to low resolution data along the PE direction in ACS image ("blip-down") and the resulting field map. This low resolution can be improved by acquiring k-space ACS data in a partial-Fourier fashion and reconstructing the ACS image using POCS.
The proposed pipeline is particularly valuable for a long EPI protocol composed of multiple sequences, such as microstructural imaging using multi-shell dMRI, where at least a b=0 image and its ACS data are acquired in the beginning of each sequence. The inter-sequence motion can be easily accounted for by the ACS scan of each sequence, lifting the requirement of multiple "blip-down" image acquisitions.
Acknowledgements
We would like to thank Els Fieremans and Dmitry Novikov for sharing the in vivo MRI data in ref. [12], and Thorsten Feiweier for the discussion of EPI and PI in WIP and clinical sequences. Research was supported by the Office of the Director of the NIH under award DP5 OD031854, by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under award U01 EB026996, R01 EB028797, R03 EB031175, U01 EB025162, P41 EB030006, and the NVidia Corporation for computing support.References
1. Stehling, M. K., Turner, R., & Mansfield, P. (1991). Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science, 254(5028), 43-50.
2. Andersson, J. L., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 20(2), 870-888.
3. Skare, S., Newbould, R. D., Clayton, D. B., Albers, G. W., Nagle, S., & Bammer, R. (2007). Clinical multishot DW‐EPI through parallel imaging with considerations of susceptibility, motion, and noise. Magnetic Resonance in Medicine, 57(5), 881-890.
4. Kellner, E., Dhital, B., Kiselev, V. G., & Reisert, M. (2016). Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic resonance in medicine, 76(5), 1574-1581.
5. Andersson, J. L., Jenkinson, M., & Smith, S. (2007). Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford, 2(1), e21.
6. Andersson, J. L., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, 1063-1078.
7. Sotiropoulos, S. N., Jbabdi, S., Xu, J., Andersson, J. L., Moeller, S., Auerbach, E. J., ... & Wu-Minn Hcp Consortium. (2013). Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage, 80, 125-143.
8. Alfaro-Almagro, F., Jenkinson, M., Bangerter, N. K., Andersson, J. L., Griffanti, L., Douaud, G., ... & Smith, S. M. (2018). Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage, 166, 400-424.
9. Casey, B. J., Cannonier, T., Conley, M. I., Cohen, A. O., Barch, D. M., Heitzeg, M. M., ... & Dale, A. M. (2018). The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience, 32, 43-54.
10. Basser, P. J., Mattiello, J., & LeBihan, D. (1994). Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B, 103(3), 247-254.Chicago
11. Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H., & Kaczynski, K. (2005). Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine, 53(6), 1432-1440.
12. Lee, H. H., Novikov, D. S., & Fieremans, E. (2021). Removal of partial Fourier‐induced Gibbs (RPG) ringing artifacts in MRI. Magnetic Resonance in Medicine.
Figures