4854
Development and evaluation of a comprehensive array of gray matter labels for the MIITRA atlas: Interoperability with complementary atlases1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
The Multichannel Illinois Institute of Technology & Rush university Aging (MIITRA) atlas constructed using high quality MRI data on a large (N=400), diverse, community cohort of non-demented older adults, contains high resolution (0.5mm isotropic) structural and diffusion tensor imaging templates. The purpose of this work was to build and evaluate a comprehensive array of gyral-based, cytoarchitecture-based, and functional connectivity-based gray matter labels in MIITRA space in order to enhance the functionality of the MIITRA atlas and its interoperability with complementary atlases.
INTRODUCTION
The Multichannel Illinois Institute of Technology & Rush university Aging (MIITRA)[1] atlas constructed using high quality MRI data on a large (N=400), diverse, community cohort of non-demented older adults, contains high resolution (0.5mm) structural and diffusion tensor imaging templates. The purpose of this work was to build and evaluate a comprehensive array of gyral-based, cytoarchitecture-based, and functional connectivity-based gray matter labels in MIITRA space in order to enhance the functionality of the MIITRA atlas and its interoperability with complementary atlases. [KA1]Reference Ridwan’s paperMETHODS
Data and preprocessing:T1w images from the 400 older adults included in the construction of the MIITRA atlas (50% male; 64.9-98.9 years of age; 54% white, 43% black) were processed with Freesurfer’s[2] standard recon-all pipeline. The Freesurfer outputs for all images were manually edited. Parcellations from Yeo[3], Desikan-Killiany[4], HCP-MMP[5], Brainnetome[6], Brodmann[7], Destrieux[8], Campbell[9], Flechsig[10], Mindboggle[11], Kleist[12], Smith[13], and EconomoCT[14,15] atlases were projected onto the surface of each image and then converted to volumetric labels for each image. The T1w images were also nonlinearly registered using ANTs[16] SyN to the MNI152 6th gen[17], MNI Colin[18], and the MNI 2009b[19] templates, and volumetric labels from Harvard-Oxford[20], Julich[21], AAL3[22], Buckner[23], CoBrALab[24], CAREN[25], Striatum subdivision[26] and Hippocampus subdivisions[26] atlases were warped to the space of each image. The same approach was followed for a separate group of 100 older adults participating in the same studies of aging as the persons included in MIITRA (age-range 65-95, male-female ratio 40:60) to serve as reference labels in the evaluation process.
Construction of gray matter labels
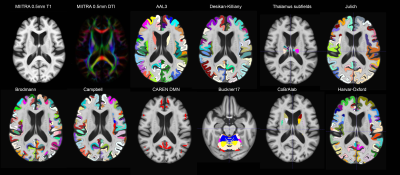
The gray matter labels of the 400 individuals included in the development of the MIITRA atlas were transformed from raw space to exact physical locations in MIITRA space using the ANTs-derived forward transformations that were applied on individual T1w images to build the MIITRA T1w template[27]. The label of a 0.5mm isotropic voxel in MIITRA space was then calculated using majority voting among all the labels that were mapped to that voxel. This technique was applied to every set of labels mentioned above, generating a comprehensive array of complementary gray matter labels in MIITRA space (Fig.1).
Evaluation
T1w images from the 100 older adults of the evaluation group were nonlinearly normalized to the 0.5mm MIITRA T1w template and the inverse transformation was applied to the gray matter labels of the MIITRA atlas to transform them to each individual’s space. For each individual, the warped gray matter labels from the MIITRA atlas were compared to the corresponding reference labels. The comparison was conducted within an individual’s gray matter mask. First, the overlap between warped and reference labels was evaluated using the Dice coefficient, Jaccard coefficient, sensitivity, and specificity. Next, the geometry of the labels was compared using the average volume of each label. Then the label dissimilarity was assessed using the volume error.
RESULTS
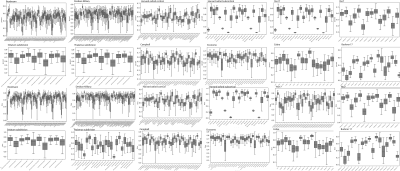
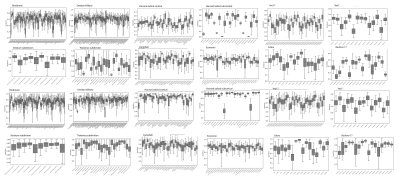
Examples of the gray matter labels in MIITRA space are shown in Figure 1. The measures of overlap between the warped and reference labels for each set of labels were generally high, with an average Dice coefficient of 0.78±0.3, average Jaccard coefficient of 0.59±0.27 (Fig.2), sensitivity of 0.74±0.1, and specificity of 0.86±0.35 (Fig.3). The geometry of the warped labels showed a high correlation with the geometry of the reference labels both in terms of the average volume of each label (correlation coefficient = 0.997, p-value < 10-10) (Fig.4). The values of dissimilarity between the warped and reference labels were low, with an average volume error of 0.38±0.2.DISCUSSION
A comprehensive array of complementary gray matter labels were constructed for the MIITRA atlas in this work. These labels include gyral-based, cytoarchitecture-based, and functional connectivity-based labels which will enhance the functionality of the MIITRA atlas. The new labels will also enhance the interoperability of MIITRA with the source atlases. In the evaluation, the gray matter labels in the MIITRA atlas showed high overlap, high geometric correlation, and low dissimilarity with the reference labels, demonstrating that there is a high agreement between the labels obtained from the MIITRA atlas and the reference labels from the source atlases.CONCLUSION
This work developed a comprehensive array of complementary gray matter labels for the MIITRA atlas. These labels, in combination with the high-resolution templates of the atlas, may become particularly useful resources in neuroimaging studies of the aging brain.Acknowledgements
This study was supported by:
National Institute on Aging (NIA) R01AG052200
National Institute on Aging (NIA) P30AG010161
National Institute on Aging (NIA) P30AG072975
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) RF1AG022018
National Institute on Aging (NIA) R01AG056405
References
1. Ridwan AR, Niaz MR, Wu Y, et al. Development and evaluation of a high performance T1-weighted brain template for use in studies on older adults. Hum Brain Mapp. 2021;42(6):1758-1776
2. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781.
3. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125-1165. doi:10.1152/jn.00338.2011
4. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 Jul 1;31(3):968-80
5. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171-178. doi:10.1038/nature18933
6. Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26(8):3508-3526.
7. K. Brodmann. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien dargestellt Auf Grund des Zellenbaues
Barth (1909)
8. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1-15. doi:10.1016/j.neuroimage.2010.06.010
9. M., F. Localisation of Cerebral Functions . Nature 74, iii–v (1906).
10. P.E. Flechsig. Anatomie Des Menschlichen Gehirns und Rückenmarks auf Myelogenetischer Grundlage. G. Thieme (1920)
11. 101 labeled brain images and a consistent human cortical labeling protocol
Arno Klein, Jason Tourville. Frontiers in Brain Imaging Methods. 6:171
12. Kleist K. (1934) Gehirnpathologie vornehmlich auf Grund der Kriegserfahrungen (Leipzig: Barth).
13. G.E. Smith. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol., 41 (Pt 4) (1907), p. 237
14. C.F. von Economo, G.N. Koskinas. Die Cytoarchitektonik Der Hirnrinde Des Erwachsenen Menschen. J. Springer (1925)
15. Rory Pijnenburg, Lianne H. Scholtens, Dirk Jan Ardesch, Siemon C. de Lange, Yongbin Wei, Martijn P. van den Heuvel,. Myelo- and cytoarchitectonic microstructural and functional human cortical atlases reconstructed in common MRI space, NeuroImage,
16. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011 Feb 1;54(3):2033-44.
17. Mazziotta, J.C., Toga, A.W., Evans, A.C., Fox, P., Lancaster, J., 1995. A probabilistic atlas of the human brain: theory and rationale for its development. NeuroImage 2, 89–101
18. Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. “Enhancement of MR images using registration for signal averaging.” J Comput Assist Tomogr. 1998 Mar-Apr;22(2):324–33.
19. VS Fonov, AC Evans, K Botteron, CR Almli, RC McKinstry, DL Collins and BDCG, Unbiased average age-appropriate atlases for pediatric studies, NeuroImage,Volume 54, Issue 1, January 2011, ISSN 1053–8119
20. Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006 Apr;83(2-3):155-71
21. Amunts K, Mohlberg H, Bludau S, Zilles K. Julich-Brain: A 3D probabilistic atlas of the human brain's cytoarchitecture. Science. 2020;369(6506):988-992.
22. Edmund T. Rolls, Chu-Chung Huang, Ching-Po Lin, Jianfeng Feng, Marc Joliot, Automated anatomical labelling atlas 3, NeuroImage, Volume 206, 2020, 116189.
23. Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C., Yeo, B. T. T., & Yeo, T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of neurophysiology, 2322-45.
Figures