4850
INTRACRANIAL EFFECTS OF ARTIFICIAL GRAVITY: A 3T MRI STUDY1Diagnostic Imaging, UTSHC-Houston, Houston, TX, United States, 2UTSHC-Houston, Houston, TX, United States, 3NASA, Houston, TX, United States, 4KBR, Houston, TX, United States, 5BCM, Houston, TX, United States
Synopsis
Headward fluid shift is a natural consequence of working in microgravity. It is theorized to be the cause of changes in brain morphology and the development of optic nerve pathology in astronauts. This study looked at the application of artificial gravity in the form of short-arm centrifugation at 0.3g as a potential countermeasure in 24 healthy volunteers over a 60 day period. Headward fluid shift was simulated using a head down tilt bed rest model. Both continuous and intermittent artificial gravity for 30 minutes per day failed to show any benefit on intracranial changes associated with chronic headward fluid shift.
Introduction
Spaceflight associated neuro-ocular syndrome (SANS) is characterized by the development of optic disc edema, posterior globe flattening, choroidal/retinal folds and hyperopic refractive errors1. SANS is hypothesized to be a result of headward fluid shifts that invariably occurs in the microgravity environment. As a countermeasure, artificial gravity (AG) through centrifugation has been proposed to reduce headward fluid shift, however there is no current proof of benefit. The goal of this study was determine if the application of AG can prevent or reduce known changes in brain volumetry, internal carotid artery (ICA) stroke volume and cerebral spinal fluid (CSF) flow velocity that occur during simulated chronic headward fluid shift using head down tilt bed rest (HDTBR) methodology2 as an indicator of countermeasure efficacy.Methods
Healthy volunteers were recruited for an IRB approved HDTBR study performed at the German Aerospace Center in Cologne, Germany. Strict six-degree HDTBR was used as a spaceflight analog to induce a continuous headward fluid shift. HDTBR was carried out for 60 days for all subjects. Short-arm centrifugation was utilized to generate AG equating to approximately 0.3g of acceleration at the level of the eye. The subjects were divided equally into three groups: No AG (control; n=8), daily intermittent AG (6 x 5 min iAG; n=8), and daily continuous 30 min cAG (n=8). All studies were performed on a single dedicated 3T MRI Scanner. Pulse-gated MRI phase-contrast flow imaging was used to quantify ICA stroke volume and peak-to-peak CSF flow velocity in the mid cerebral aqueduct. 3D-SPGR was acquired for volumetric segmentation of the brain and CSF spaces. MRI acquisitions were obtained at baseline (BDC), 14 days into HDTBR (HDTBR14), 52 days into HDTBR (HDTBR52) and 3-5 days after HDTBR (recovery, R+3/5). The data were analyzed by the mixed model, which included intervention and time (BDC, HDTBR 14, HDTBR 52, R+3/5) as the fixed effects and included subject as the random effect.Results
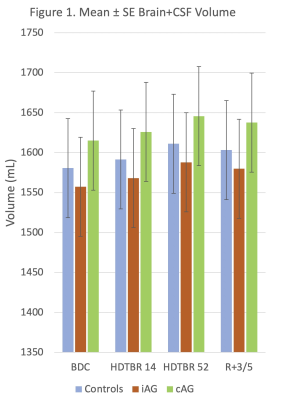
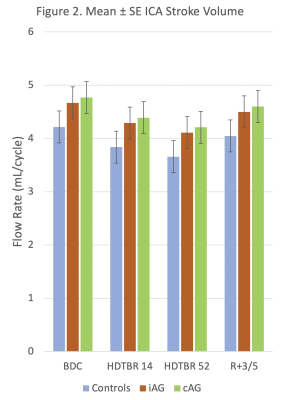
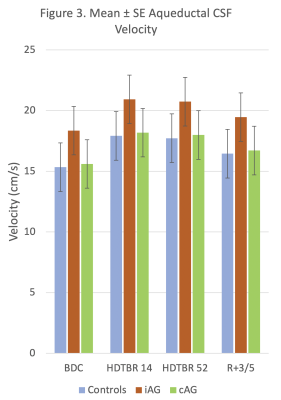
24 volunteers (16 men, 8 women, mean age = 33 years ± 9 [standard deviation] and mean BMI = 24.3 kg/m2 ± 2.0) successfully completed all phases of the study. Strict six-degree HDTBR was characterized by progressive increases in combined brain and CSF volumes and progressive decreases in mean ICA stroke volume from baseline to 52 days post intervention (P<.01) (Figs. 1-2). Overall, mean CSF peak-to-peak flow velocity increased from baseline to 52 days post intervention (P<.01), reaching its maximum value at HDTBR14 (Fig. 3). Compared to baseline, only combined brain and CSF volumes did not return to baseline values in the recovery period (P<.01). Neither iAG nor cAG exerted any significant effects on the measured MRI brain parameters as compared to HDTBR alone (P=NS).Conclusion
Our results indicate that HDTBR at 6-degree was effective in producing alterations in ICA stroke volume, aqueductal CSF flow velocity and combined brain and CSF volumetric change that is associated with chronic headward fluid shift. Short duration, 30-min daily exposure to either iAG or cAG appears to be insufficient in preventing or reducing the effects of chronic HDTBR and thus may not be a suitable countermeasure as currently deployed. AG protocol modification including increased duration and magnitude of exposure should be considered for future research.Acknowledgements
NASA Human Research ProgramReferences
(1) Lee AG, Mader TH, Gibson CR, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity 2020;6:7. DOI: 10.1038/s41526-020-0097-9.2.
(2) Kramer LA, Hasan KM, Sargsyan AE, et al. Quantitative MRI volumetry, diffusivity, cerebrovascular flow, and cranial hydrodynamics during head-down tilt and hypercapnia: the SPACECOT study. J Appl Physiol (1985) 2017;122(5):1155-1166. DOI: 10.1152/japplphysiol.00887.2016.
Figures