4848
Systemic inflammation alters brain blood flow and R2*: An in-vivo 9.4T MRI animal study1Department of Radiology, University of Calgary, Calgary, AB, Canada
Synopsis
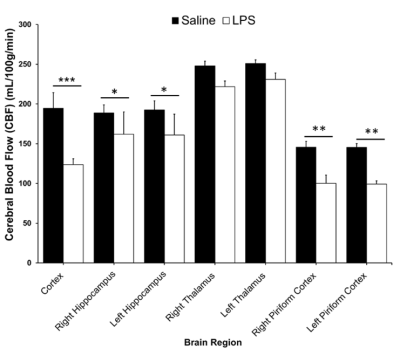
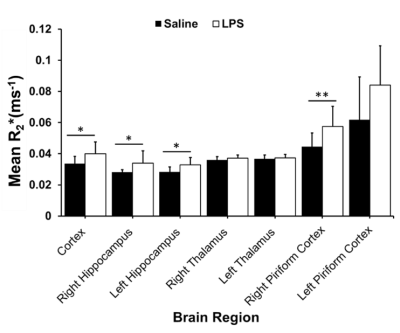
Systemic inflammation is linked to a range of neurological diseases. Reductions in cerebral blood flow (CBF) and the presence of brain hypoxia have been detected in animal models of inflammation and in multiple sclerosis, a disease with significant inflammation. Reduced CBF combined with hypoxia could exacerbate damage in neuroinflammatory conditions. To study this link, we used in-vivo 9.4T MRI to quantify CBF and R2*, a marker of deoxyhemoglobin, following systemic inflammation induced by bacterial lipopolysaccharide. We found reduced CBF and increased R2* in 4 regions, including the cortex and hippocampus—indicating that inflammation is accompanied by hypoxia and reduced CBF.
Introduction
Systemic inflammation can induce neuroinflammation and is associated with an array of neurological conditions, such as COVID-19 and multiple sclerosis (MS). We proposed that inflammation might be linked to reductions in cerebral blood flow (CBF) and brain hypoxia (low oxygenation)1. Reductions in CBF have also been detected in people with MS2 and it was hypothesized that this reduction could be linked to inflammation1, 2. We detected brain hypoxia using NIRS in MS participants3 and in an inflammatory mouse model known as experimental autoimmune encephalomyelitis (EAE)4. These data provide evidence of a relationship between inflammation and brain hypoxia. We propose that inflammation-associated impairments in CBF can reduce oxygen delivery to the brain, which could contribute to the development of brain hypoxia. To advance our understanding of the link between inflammation, CBF, and hypoxia, we used 9.4T MRI to quantify CBF and relaxation rate (R2*), a qualitative marker of deoxyhemoglobin, in the bacterial lipopolysaccharide (LPS) mouse model of systemic inflammation.Methods
Female C57BL/6 mice were randomly divided into saline (n=10) or 2 mg/kg LPS (n=11) groups. Mice were injected via intraperitoneal injection to induce systemic inflammation. Injections were repeated once daily for 3 days. Three hours after the third injection, we performed in-vivo 9.4T MRI. We assess CBF using a continuous arterial spin labeling (CASL) sequence (TR = 3000ms, TE = 2.7ms, TEeff = 13.5 ms, averages: 16, RARE factor = 36, matrix = 128x128, FOV = 25.6 x 25.6 mm). The T1 map was obtained using a RARE-VTR sequence: TR=100, 500, 1000, 3000, 7500 ms, TE=10 ms. R2* was determined as the inverse of T2*, collected using a multi-gradient echo (MGE) sequence (TR = 1500 ms, TE: 3.1 ms, echo spacing = 4 ms, averages = 8, slices = 12, matrix = 128x128, FOV = 25.6 x 25.6 mm). Mice were sacrificed following MRI and brains extracted for histological analysis of inflammation. Leukocyte staining was performed using CD45 and blood vessels were stained using CD31. CBF and R2* were quantified in 7 brain regions. A Student’s t-test was used to compare CBF and R2* between control and LPS treated groups with an FDR-correction (Benjamini-Hochberg) for multiple comparisons. A Pearson’s correlation was used to assess the relationship between CBF and R2*.Results
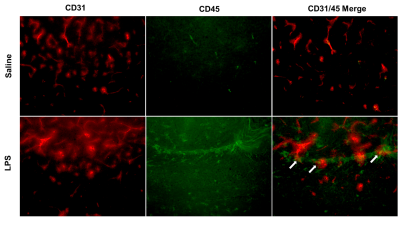
In the LPS-treated group, we detected reductions in CBF in the cortex (p<0.001), right and left hippocampus (p<0.03), right piriform cortex (p<0.01), and left piriform cortex (p<0.001) (Figure 1). LPS also resulted in increases in R2* in the cortex (p<0.05), right and left hippocampus (p<0.05), and right piriform cortex (p<0.01) (Figure 2). R2* increase is consistent with increase in tissue deoxyhemoglobin (dHb). CBF and R2* were negatively correlated (p<0.05, r = -0.54) in the cortex. Our preliminary histological staining appears to show more CD45 positive leukocytes in the hippocampus of the LPS treated mouse as compared to the saline, as well as more accumulation of CD45 positive leukocytes within the hippocampal blood vessels (Figure 3).Discussion
Using in-vivo 9.4T MRI, we demonstrated a reduction in CBF and an increase in R2* in the LPS model. An increase in R2* is consistent with an increase in deoxyhemoglobin, which would be a non-invasive marker of increased hypoxia within the brain. Our data suggest that systemic inflammation can cause a reduction in CBF and a consequent increase in brain hypoxia. The mechanism by which CBF reduction occurs is unclear. Preliminary results from CD45 (leukocytes) and CD31 (blood vessels) staining suggest that leukocytes might be aggregating inside small blood vessels within the hippocampus. A similar observation has been made previously in a single-dose 5 mg/kg LPS rat model, where it was found that macrophages accumulated inside the blood vessels of the cortex and the choroid plexus following LPS treatment5. Based on these findings, it is possible that cerebral blood vessels can become physically occluded by immune cells in response to a highly inflammatory condition, such as the one we expect in the LPS model, or in conditions such as COVID-19, and this blockage of blood vessels might be contributing to CBF reduction, and brain hypoxia.Conclusion
Systemic inflammation decreased CBF and increased brain R2*/deoxyhemoglobin, which is consistent with inflammation causing hypoxia. These findings have implications for neurological diseases such as MS, which have an inflammatory component. Such inflammation could cause a hypoxia-inflammation cycle which could increase neurological dysfunction and damage.Acknowledgements
This work was funded by Natural Sciences and Engineering Research Council (RGPIN-2015-06517), Canadian Foundation for Innovation, and Brain Canada. QS received undergraduate funding from Alberta MS Network, UCalgary Biomedical Engineering, and Alberta Innovates Health Solutions.References
1. Yang R and Dunn JF. Multiple sclerosis disease progression: Contributions from a hypoxia-inflammation cycle. Mult Scler. 2019; 25: 1715-8.
2. D'Haeseleer M, Beelen R, Fierens Y, et al. Cerebral hypoperfusion in multiple sclerosis is reversible and mediated by endothelin-1. Proc Natl Acad Sci U S A. 2013; 110: 5654-8.
3. Yang R and Dunn JF. Reduced cortical microvascular oxygenation in multiple sclerosis: a blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Scientific Reports. 2015; 5: 16477.
4. Johnson TW, Wu Y, Nathoo N, Rogers JA, Wee Yong V and Dunn JF. Gray Matter Hypoxia in the Brain of the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. PLOS ONE. 2016; 11: e0167196.
5. Buttini M, Limonta S and Boddeke HW. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochemistry international. 1996; 29: 25-35.
Figures