4839
Assessment of human brain pyruvate oxidation using functional hyperpolarized 13C MRS
Maheen Zaidi1,2,3, Binu P. Thomas1,4, Junjie Ma1, Salvador Pena1, Jun Chen1, James Ratnaker1, Craig R. Malloy1,4,5, Brenda Bartnik-Olson6, and Jae Mo Park1,4,7
1Advanced Imaging Research Center, UT Southwestern, Dallas, TX, United States, 2Psychology, UT Dallas, Richardson, TX, United States, 3Neurobiology, UT Dallas, Richardson, TX, United States, 4Radiology, UT Southwestern, Dallas, TX, United States, 5Internal Medicine, UT Southwestern, Dallas, TX, United States, 6Radiology, Loma Linda University, Loma Linda, CA, United States, 7Electrical and Computer Engineering, UT Dallas, Richardson, TX, United States
1Advanced Imaging Research Center, UT Southwestern, Dallas, TX, United States, 2Psychology, UT Dallas, Richardson, TX, United States, 3Neurobiology, UT Dallas, Richardson, TX, United States, 4Radiology, UT Southwestern, Dallas, TX, United States, 5Internal Medicine, UT Southwestern, Dallas, TX, United States, 6Radiology, Loma Linda University, Loma Linda, CA, United States, 7Electrical and Computer Engineering, UT Dallas, Richardson, TX, United States
Synopsis
[13C]Bicarbonate production from hyperpolarized [1-13C]pyruvate in the brain is directly related to pyruvate oxidation and, thus serves a potential biomarker of brain function. In this study, we assessed time-wise bicarbonate production in the visual cortex during activation with minimal perturbation of its precursors, pyruvate and lactate, using a multichannel 13C receive array, a spectral-spatial RF pulse that fully excites bicarbonate signals, and dynamic 13C MRS. The real-time changes of bicarbonate production in response to visual stimuli were observed in healthy volunteers.
Background
A series of metabolic processes are associated with neuronal activation, such as increases in blood flow, oxygen and glucose consumption, and oxidative phosphorylation. These processes are essential to meet increased energy needs for the required brain function. Pyruvate dehydrogenase (PDH), a mitochondrial enzyme that regulates pyruvate flux into the mitochondria and the subsequent tricarboxylic acid (TCA) cycle, is the main pathway for oxidative phosphorylation. Blood oxygen level-dependent (BOLD) fMRI detects neuronal activation, based on changes in magnetic susceptibility due to deoxygenation of hemoglobin, and has been the dominant methodology for studying brain activation. However, BOLD fMRI depends on signal changes that are several steps away from neuronal activation and thus, is susceptible to other physiological changes and artifacts, which can result in diluted signal sensitivity. In addition, the temporal resolution is limited due to the slow hemodynamic response. The advent of dissolution dynamic nuclear polarization (DNP) provides an opportunity to assess oxidative phosphorylation as the production of [13C]bicarbonate from administered hyperpolarized (HP) [1-13C]pyruvate is a direct biomarker of PDH activity. Recent translation of the technique in human brains demonstrated that [13C]bicarbonate is primarily produced in cerebral cortex (1-3), with the absence of pyruvate carboxylase-specific products suggesting that bicarbonate is primarily produced from neurons (3). We hypothesized that HP [13C]bicarbonate production in the brain reflects the metabolic changes associated with neuronal activation (Figure 1). In this study, we report bicarbonate production in response to visual stimulation in the visual cortex of healthy adults.Methods
The imaging protocol was approved by the local Institutional Review Board (IRB#: STU-2018-0013). All studies were performed using a clinical SPINlabTM polarizer (GE Healthcare) and a 3T MRI scanner (GE Healthcare, 750w Discovery). Three healthy volunteers (age: 22 – 45, one male and two female) were recruited. Each participant underwent a 1H/13C-integrated MR protocol (~1hr), which includes 1H BOLD fMRI, 1H MPRAGE, and two injections of HP [1-13C]pyruvate (pyruvate concentration = 250 mM, dose = 0.1 mmol/kg body weight, injection rate = 5mL/s) with a 30-min interval between injections (Figure 2A). During the first 45 min, a dual-frequency RF head coil (Clinical MR Solutions, LLC), composed of 1H quadrature Tx/Rx, 13C quadrature Tx and 8-channel 13C receive array, was used (3,4). The first HP pyruvate injection occurred after a fast GRE localizer and 2D T2-weighted FLAIR scan. 13C spectra were acquired from a brain slice including the visual cortex using a time-resolved 13C MRS sequence (TR = 4 s, spectral width = 10,000 Hz, spectral points = 4096, scan time = 4 min, slice thickness = 3 cm). The center frequencies were set on the [1- 13C]pyruvate resonance. A spectral-spatial RF pulse that excites bicarbonate with 90°, lactate with 10°, and pyruvate with 5° (Figure 2C) to fully sample bicarbonate signals within each timepoint without perturbing its precursors. During the MRS acquisition, subjects were asked to focus on an alternating black screen with a cross in the center (20 s) and circular checkerboard stimuli (4 Hz, 20 s) (Figure 2B). The second HP injection was performed without the visual activation. Between the two HP pyruvate scans, a 2D 1H BOLD fMRI with the visual stimulation and 3D 1H MPRAGE images were acquired. The BOLD fMRI and MPRAGE scans were repeated using a 24-ch 1H head coil (GE Healthcare). All clinical fluid paths were prepared in a sterile environment and the HP pyruvate solutions passed a quality control analysis prior to the injection, as previously described (3). 13C data was reconstructed in absorption mode with pyruvate, lactate, and bicarbonate quantified by integrating the peaks in each time point to display their time courses. The BOLD fMRI data were analyzed using Statistical Parametric Mapping software (SPM12; Functional Imaging Laboratory, University College London) and the resultant brain activation map (p < 0.05) overlaid on the MPRAGE images.Results and Discussion
Due to the proximity to the visual cortex, 13C data from channels #2 and #3 of the 13C receive arrays were selectively combined and analyzed (Figure 3A). Participants #1 (23 y.o., male) and #3 (22 y.o., female) showed markedly increased bicarbonate production relative to the pyruvate in response to the stimuli whereas participant #2 (45 y.o., female) showed no change in bicarbonate production. Overall, bicarbonate signal intensity relative to pyruvate peak (Bic/Pyr) in time-averaged (0 – 90 s) 13C spectra were 22.1 ± 24.2 % higher with visual stimulation than without stimulation (Figure 3B). Increased bicarbonate production was detected during the first stimulation (20 – 40 s), probably due to the high signal sensitivity (Figure 3C). BOLD fMRI confirmed the regional activation of BOLD signal in the visual cortex (Figure 4).Conclusion
In this study, we observed increased PDH activity of the human visual cortex in response to visual stimuli. We plan to expand the study to a larger number of subjects and develop analytical methods.Acknowledgements
Personnel Support: We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern for recruiting and imaging the volunteers – Jeannie Baxter, R.N. and Kelley Derner, R.N.Funding: National Institutes of Health of the United States (R01 NS107409, P41 EB015908, S10 OD018468, S10 RR029119); The Welch Foundation (I-2009-20190330).
References
1. Grist JT, McLean MA, Riemer F, Schulte RF, Deen SS, Zaccagna F, Woitek R, Daniels CJ, Kaggie JD, Matyz T, Patterson I, Slough R, Gill AB, Chhabra A, Eichenberger R, Laurent M-C, Comment A, Gillard JH, Coles AJ, Tyler DJ, Wilkinson I, Basu B, Lomas DJ, Graves MJ, Brindle KM, Gallagher FA. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. Neuroimage. 2019 Jan 11;189:171–9. PMCID: PMC64351022. Gordon JW, Chen H-Y, Autry A, Park I, Van Criekinge M, Mammoli D, Milshteyn E, Bok R, Xu D, Li Y, Aggarwal R, Chang S, Slater JB, Ferrone M, Nelson S, Kurhanewicz J, Larson PEZ, Vigneron DB. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med. 2019 Apr;81(4):2702–9. PMCID: PMC6372313
3. Ma J, Pinho MC, Harrison CE, Chen J, Sun C, Hackett EP, Liticker J, Ratnakar J, Reed GD, Chen AP, Sherry AD, Malloy CR, Wright SM, Madden CJ, Park JM. Dynamic 13 C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn Reson Med. 2021 Oct 22. PMID: 34687086
4. Park JM, Harrison CE, Ma J, Chen J, Ratnakar J, Zun Z, Liticker J, Reed GD, Chhabra A, Haller RG, Jue T, Malloy CR. Hyperpolarized 13C MR Spectroscopy Depicts in Vivo Effect of Exercise on Pyruvate Metabolism in Human Skeletal Muscle. Radiology. 2021 Jun 22;:204500. PMCID: PMC8409104
Figures
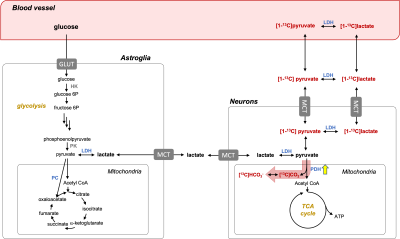
Figure 1. Detection of PDH activation in the brain using HP [1-13C]pyruvate. HP pyruvate enters neurons and astroglia either as pyruvate or lactate. Unlike PC-dominated pyruvate metabolism in astroglia, PDH is the primary pathway in neurons for pyruvate to enter the mitochondria. The underlying hypothesis is that PDH flux into the mitochondria is upregulated in response to neuronal activation, resulting an increase of HP bicarbonate (HCO3-) production. PDH: pyruvate dehydrogenase; LDH: lactate dehydrogenase; TCA: tricarboxylic acid; PC: pyruvate carboxylase.
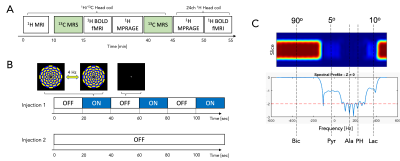
Figure 2. Study protocol. (A) Overall MR protocol that includes two injections of HP [1-13C]pyruvate and 1H BOLD fMRI. (B) A sequence of black screen and alternating circular checkerboard stimuli were projected for the first 13C scan and the BOLD fMRI scans. (C) Spectral spatial RF pulse that fully excites bicarbonate and minimally samples lactate and pyruvate was used for 13C MRS acquisitions.
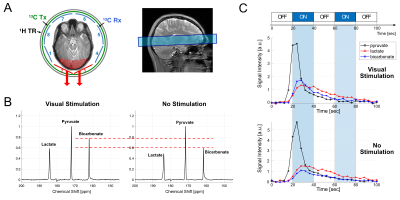
Figure 3. Functional 13C MRS. (A) Slice prescription of 13C MRS and the selection of 13C receive arrays for analysis. (B) Representative time- averaged 13C spectra (channel #2 and #3 only) from participant #1 with and without visual stimulation. (C) Time courses of hyperpolarized [1- 13C]pyruvate, [1-13C]lactate and [13C]bicarbonate, which were excited by 5°, 10°, and 90°, respectively, per time point.
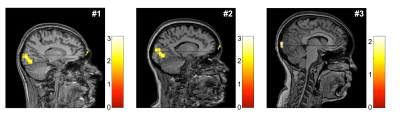
Figure 4. Brain activation maps from BOLD fMRI. The brain activation maps are overlaid on the MPRAGE images acquired using 24-ch 1H head coil. The study participants showed activated regions in the visual cortex in response to the visual stimuli.
DOI: https://doi.org/10.58530/2022/4839