4838
Pathway analysis of cerebral bicarbonate production from hyperpolarized [1-13C]pyruvate1Advanced Imaging Research Center, UT Southwestern, Dallas, TX, United States, 2Psychology, UT Dallas, Richardson, TX, United States, 3Neurobiology, UT Dallas, Richardson, TX, United States, 4Radiology, Loma Linda University, Loma Linda, CA, United States, 5Radiology, UT Southwestern, Dallas, TX, United States, 6Electrical Engineering, UT Dallas, Richardson, TX, United States
Synopsis
Detection of [13C]bicarbonate in the brain from hyperpolarized [1-13C]pyruvate provides a unique opportunity for assessing cerebral mitochondrial function in-vivo. Although decarboxylation of the labeled pyruvate occurs within the mitochondria, the metabolic path of the injected pyruvate could be complex before being transported to intracellular space. In this study, we investigated the likelihood of pyruvate being converted to lactate prior to decarboxylation.
Introduction
[1-13C]Lactate and [13C]bicarbonate are products of hyperpolarized (HP) [1-13C]pyruvate metabolism in the brain (1). Unlike [1-13C]lactate, production of [13C]bicarbonate is mitochondrion-specific and pyruvate dehydrogenase (PDH), the enzyme that catalyzes the decarboxylation of pyruvate to acetyl-CoA, is considered to be the dominant pathway for the bicarbonate production (2). Detection of [13C]bicarbonate, therefore, provides an exciting opportunity to assess cerebral mitochondrial function and metabolism in-vivo (1). While intracellular pyruvate is undoubtedly the direct precursor of bicarbonate, it has been often assumed that this pyruvate is directly from administered HP pyruvate bolus, despite traveling through several biomembranes in the brain. However, lactate may play a significant role in the production of bicarbonate since the interconversion of pyruvate and lactate, catalyzed by the enzyme lactate dehydrogenase (LDH), is rapid and can occur before and after reaching brain tissues (3) (Figure 1). The contribution of pyruvate-lactate interconversion to cerebral [13C]bicarbonate production from [13C]pyruvate is narrowly explored. In this study, we present evidence supporting that HP [13C]lactate derived from HP [1-13C]pyruvate is a significant contributor to [13C]bicarbonate production in the brain.Methods
We conducted three sets of experiments for the study. First, 13C NMR isotopomer analysis was performed in rats (n = 6, 240.0 ± 88.0 g) to compare the metabolic fates of [U-13C]pyruvate or [U-13C]lactate following bolus injection (0.6 mmol/kg in 60-mM solution) to mimic HP studies. Approximately two minutes after the injection, the rats were sacrificed, and the brain tissues were harvested and freeze-clamped with liquid nitrogen. Proton-decoupled 13C spectra of perchloric acid extracts were acquired using a 14.1-T NMR spectrometer (Bruker) as previously described (4) and analyzed using TopSpin (Bruker) to calculate the fractional enrichments of lactate, glutamate, and succinate. In a second group of rats (n = 3, 147 ± 20 g), in vivo 13C MRS was acquired using a 3T 750w MRI scanner (GE Healthcare) and a custom-made 13C surface coil (diameter = 28 mm), immediately following a bolus injection of 60-mM HP [1-13C]pyruvate followed by a second injection of 60-mM HP [1-13C]lactate 2–4 hours later. Following each injection, dynamic 13C MRS using a slice-selective RF excitation (flip-angle = 10°, slice = 15 mm, TR = 3s) was performed. Sample preparation and polarization procedure are described in (5). Products-to-total 13C (TC) and bicarbonate-to-TC were calculated and compared between lactate and pyruvate injected groups. Finally, time-resolved 13C spectra were acquired from a healthy volunteer (60 y.o, female, BMI = 29.4) using a 13C/1H dual-frequency head coil (Clinical MR Solutions) with two consecutive injections of HP [1-13C]pyruvate as previously described (2). Two spectral-spatial RF pulses were used; one RF pulse excited 90° on bicarbonate, 5° on pyruvate and 90° on lactate whereas the other was designed to excite 10° on lactate.Results and Discussion
The NMR isotopomer analysis study revealed similar 13C spectral patterns in the tissue extracts of rats injected with [U-13C]pyruvate and those with [U-13C]lactate (Figure 2). Specifically, the fractional enrichments of [4,5-13C2]glutamate and [1,2-13C2]succinate were 2.3 ± 1.4 % and 1.9 ± 0.8 % in pyruvate-injected rats and 2.2 ± 1.2 % and 1.3 ± 0.7 % in lactate- injected rats, respectively, indicating similar amounts of 13C passing through PDH between the pyruvate and lactate injections. In contrast, in vivo HP study showed that [13C]bicarbonate/TC was approximately twice the signal when [1-13C]pyruvate was injected (0.029 ± 0.003) as compared to the [1-13C]lactate injection (0.014 ± 0.002, p = 0.013, Figure 3). This is likely due to substrate competition between the large intrinsic, unlabeled lactate pool and the injected labeled lactate pool in exchange with pyruvate before converting to bicarbonate (6). As the tissue-level concentrations of intrinsic lactate and injected lactate are largely comparable, the labeled bicarbonate becomes roughly a half when HP lactate was injected. This does not apply to 13C-pyruvate injection since the intrinsic pyruvate pool is much smaller. Significantly larger total HP products relative to TC with pyruvate (0.313 ± 0.045) than with lactate (0.036 ± 0.006, p = 0.011) can be explained in a similar manner. The discrepancy between the ex-vivo NMR and in-vivo HP findings could be due to multiple reasons such as T1 relaxation or differences in the timing of measurements. In particular, the brain tissues were collected 2 mins after a bolus injection of lactate or pyruvate in the ex vivo study whereas the majority of bicarbonate peaks were detected within 1 min from the HP injection. In the human study, much larger HP [13C]bicarbonate was detected when lactate was minimally excited (Figure 4), suggesting that a large fraction of [13C]bicarbonate is produced from [1-13C]lactate. This observation is consistent with a previous study that saturated lactate prior to bicarbonate imaging in the brain (7).Conclusion
13C-lactate generated from an HP [1-13C]pyruvate bolus is a substantial contribution to [13C]bicarbonate formation in the brain.Acknowledgements
Personnel Support: We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern for recruiting and imaging the volunteers – Jeannie Baxter, R.N. and Kelley Derner, R.N.Funding: National Institutes of Health of the United States (R01 NS107409, P41 EB015908, S10 OD018468, S10 RR029119); The Welch Foundation (I-2009-20190330).
References
-
Mayer D, Yen Y-F, Takahashi A, Josan S, Tropp J, Rutt BK, Hurd RE, Spielman DM, Pfefferbaum A. Dynamic and high-resolution metabolic imaging of hyperpolarized [1-13C]-pyruvate in the rat brain using a high-performance gradient insert. Magn Reson Med. 2011 May;65(5):1228–33.
-
Ma J, Pinho MC, Harrison CE, Chen J, Sun C, Hackett EP, Liticker J, Ratnakar J, Reed GD, Chen AP, Sherry AD, Malloy CR, Wright SM, Madden CJ, Park JM. Dynamic 13 C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn Reson Med. 2021 Oct 22. doi: 10.1002/mrm.29049
-
Xu T, Mayer D, Gu M, Yen Y-F, Josan S, Tropp J, Pfefferbaum A, Hurd R, Spielman D. Quantification of in vivo metabolic kinetics of hyperpolarized pyruvate in rat kidneys using dynamic 13C MRSI. NMR Biomed. 2011 Oct;24(8):997–1005. PMCID: PMC3169748
-
Chen J, LaGue E, Li J, Yang C, Hackett E, Mendoza M, Alger J, DeBerardinis R, Corbin IR, Billingsley KL, Park JM. Profiling Carbohydrate Metabolism in Liver and Hepatocellular Carcinoma with [13C]-Glycerate Probes. Anal Sens. 2021 Sep 14. doi: 10.1002/anse.202100034
-
Chen J, Hackett EP, Kovacs Z, Malloy CR, Park JM. Assessment of hepatic pyruvate carboxylase activity using hyperpolarized [1-13 C]-l-lactate. Magn Reson Med. 2021 Mar;85(3):1175–82.
-
Spielman DM, Park JM. Kinetic modeling of enzymatic reactions in analyzing hyperpolarized NMR data. In: Jue T, Mayer D (eds). Dynamic Hyperpolarized Nuclear Magnetic Resonance. Handbook of Modern Biophysics. Springer, Cham. 2021 May; 103-121. doi:10.1007/978-3-030-55043-1_5
Lee CY, Soliman H, Bragagnolo ND, Chen AP, Perks WJ, Heyn C, Black SE, Cunningham CH. Is [1-13C]lactate converted to 13C-bicarbonate in the human brain? Int’l Soc Magn Reson Med. 2020 May; #0698
Figures
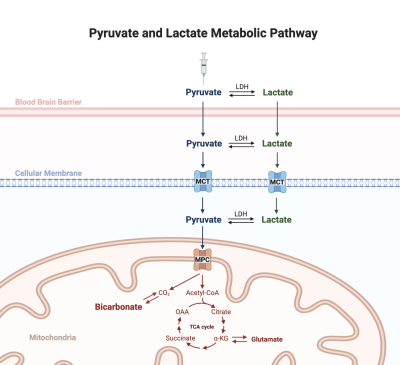
Figure 1. Metabolic pathway of HP pyruvate to produce bicarbonate. The metabolic path of the injected pyruvate could be complex before being transported to intracellular space as interconversion of pyruvate and lactate, catalyzed by the enzyme lactate dehydrogenase (LDH), is rapid and can occur before and after reaching brain tissues.
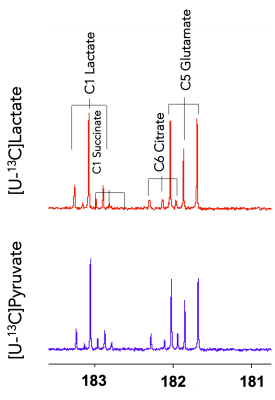
Figure 2. 13C NMR of brain tissue extracts. Brain tissues were collected from rats 2 min after a bolus injection of [U-13C]lactate (red) or [U-13C]pyruvate (blue). 13C NMR spectra of the brain extracts showed comparable fractional enrichments and labeling patterns of 13C labeling in lactate, succinate, and glutamate between lactate and pyruvate injections.
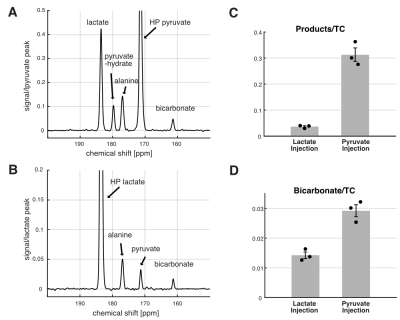
Figure 3. 13C MRS using HP [1-13C]lactate and HP [1-13C]pyruvate. Time-averaged 13C spectra of in vivo rat brains using (A) HP [1-13C]pyruvate and (B) HP [1-13C]lactate. (C) Sum of products relative to total HP 13C signals (TC) and (D) bicarbonate-to-TC were larger when HP lactate was injected than pyruvate.
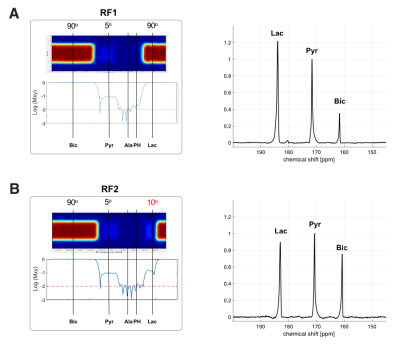
Figure 4. Effect of lactate excitation on 13C MRS in human brain. In time-averaged 13C spectrum, bicarbonate production was markedly decreased when lactate was excited with (A) 90° flip angle, compared with (B) produced bicarbonate when lactate was excited with 10° flip angle.