4822
Volumetric ultrasound hyperthermia treatment using the ExAblate body MR-guided Focused Ultrasound (MRgFUS) system1Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Radiation Oncology, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Hyperthermia refers to a cancer treatment by elevating tissue temperature to 40-45 °C. Hyperthermia can be achieved by clinical MRgFUS system, allowing real-time temperature monitoring for the precise control of hyperthermia heating. Currently the ExAblate is MR-guided FUS ablation system with the largest installation worldwide, however, requires advanced beamforming strategies for volumetric hyperthermia. This study proposed electronic beam steering, multi-focal patterns, and sector-vortex beamforming approaches in conjunction with partial array activation and was evaluated in biothermal simulations and phantom experiments. This study demonstrated the feasibility of sustained large volume heating with MR thermometry feedback using the ExAblate body system.
Introduction
Hyperthermia (HT) refers to the elevation of target temperature to 40-45 for 30-60 mins. This has been demonstrated to enhance drug delivery and/or sensitize tumors to radiation and chemotherapy.1 Hyperthermia can be achieved by a clinical MRgFUS system, allowing real-time temperature monitoring for the precise control of hyperthermia heating. The ExAblate body system (InSightec, Haifa, Israel) has been FDA approved for thermal ablation treatment of uterine fibroids and bone metastases with the largest installation worldwide.2,3 This system can potentially be used to deliver precise spatial selectivity and dynamic controlled hyperthermia to superficial and deep tissue sites. However, hyperthermia delivery with this system requires advanced beamforming strategies and technical developments to integrate a hyperthermia controller into the system. The objective of this study was to develop methods for larger volume heating using the ExAblate body system.Methods
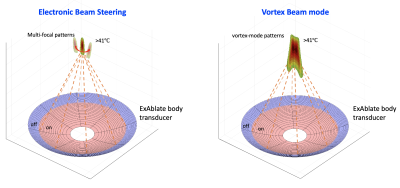
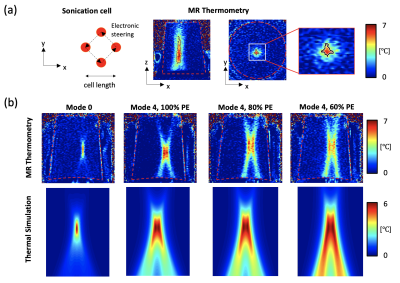
The ExAblate 2000 body system used in this study features a concentric-ring sectored-vortex phased array, with 3D positioning control and fluid coupling, integrated within the table for a 3T MRI scanner (GE Healthcare, Waukesha, WI). We developed acoustic and biothermal simulations that used a model of the array layout specific to the ExAblate system. The hybrid angular spectrum method4 was applied to calculate the 3D acoustic intensity distributions within the absorbing tissue medium. A finite element method solver of Pennes Bioheat Equation (Comsol Multiphysics 5.5) was implemented to calculate steady-state temperature distributions resulting from the generated 3D acoustic intensity distributions, with the maximum temperature set to 45 °C as appropriate for hyperthermia treatments. With this simulation, we proposed two approaches for volumetric hyperthermia using the ExAblate body system to achieve large volume heating (Fig.1): 1) using a sonication cell with rapid electronic steering. 2) with phase distributions or modes to the transducer array. In the first approach, transverse beam steering was performed to create a sonication cell, comprised of a set of four focal spots, forming a square shape in a plane perpendicular to the beam propagation axis. The second approach was based on sector-vortex beamforming that works by applying a linearly increasing phase offset to each of the sectors of the transducer. We used the phase values rotating one or more times throughout the circumference of the array. This allowed annular spaced multi-focal patterns on the cross-sectional plane, resulting in wider temperature distributions.5 To further improve the performance of volumetric HT delivery, we considered methods of reducing the effective aperture size and increasing the f-number, i.e., the ratio of the focal depth to the diameter of the transducer, by turning off a portion of the outer array. These sonication strategies were evaluated in simulations and experiments with a tissue-mimicking phantom and MR thermometry sequence at a 3T MR scanner. Images were acquired using a SPGRE sequence with a body coil (TR = 25.8 ms, TE = 12.9 ms, FOV = 280 mm 280 mm, Matrix = 256, thickness = 3 mm, and acquisition time = 3.3 s). The obtained images were transferred to an external computer via a TCP/IP connection. Proton resonance frequency shift (PRFS)-based MR thermometry was performed during heating and temperature maps were reconstructed in real-time with the field drift correction using MATLAB program (Matlab, MathWorks, Natick, MA). MR thermometry-guided hyperthermia control was integrated with the ExAblate body array to achieve uniform prolonged stable heating. The pulsed sonication mode with varying the duty cycle of the ultrasound pulses enabled the power adjustment in real-time. MR-thermometry based feedback control was performed by the power control strategy3.Results
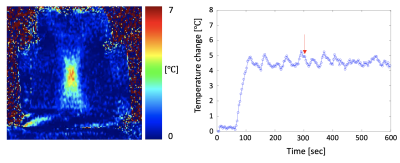
Figure 2 shows results of simulation and phantom experiments with sonication cells and sector-vortex modes. Utilizing 60% of active transducer elements allowed generating a sonication cell with an off-axis of 10 mm. Simulation and phantom experiments with MR thermometry demonstrated that sonications with mode 4 pattern and 60% and 80% array activations respectively produced the hyperthermia volume heating of 15.7 cm3 and 10.9 cm3, larger than that with the full array. Finally, the integrated hyperthermia control system based on the ExAblate body array was demonstrated to maintain stable temperature elevation for 9 mins (Fig.3).Discussion
We developed and evaluated sonication strategies for a sectored concentric ring array, designed for precise small volume thermal ablation, to generate HT over large target regions. We proposed two approaches: sonication cells with rapid beam steering and sector-vortex phasing patterns. We have also investigated reducing effective diameter of the transducer turning off a portion of the outer array elements. Our experiments showed that the focal area size and shape could be controlled by selecting sonication parameters such as mode numbers, focal depths, and the portion of active array elements according to the size of the target region, geometry, and acoustic and/or thermal properties. The acoustic and biothermal simulation framework developed in this study could be used for choosing these parameters during treatment planning.Conclusion
This study demonstrated the feasibility of large volume HT beam patterns using the ExAblate body system. As the results of experiments, both electronic beam steering and sector vortex beamforming approaches in conjunction with partial array elements were able to achieve various volume sizes of HT heating in the phantom.Acknowledgements
The authors would like to thank Noam Maimon at InSightec, Inc. for assistance with developing the techniques. This study was supported by the National Institutes of Health (NIH) under Grants R21EB026018, R21CA230120, and R01EB025990.References
1. Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742-753. doi:10.1016/j.ctrv.2015.05.009
2. Dick EA, Gedroyc WMW. ExAblate® magnetic resonance-guided focused ultrasound system in multiple body applications. Expert Review of Medical Devices. 2010;7(5):589-597. doi:10.1586/erd.10.38
3. Ozhinsky E, Salgaonkar VA, Diederich CJ, Rieke V. MR thermometry-guided ultrasound hyperthermia of user-defined regions using the ExAblate prostate ablation array. J Ther Ultrasound. 2018;6. doi:10.1186/s40349-018-0115-5
4. Vyas U, Christensen D. Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultra Ferro Freq Contl. 2012;59(6):1093-1100. doi:10.1109/TUFFC.2012.2300
5. Umemura SI, Cain CA. Analysis of temperature responses to diffused ultrasound focal fields produced by a sector-vortex phased array. Int J Hyperthermia. 1990;6(3):641-654. doi:10.3109/02656739009140960
Figures