4800
Fast adiabatic spin-echo with whole-brain ECCENTRIC for glioma metabolic imaging at 7T1University of Geneva, Geneva, Switzerland, 2CIBM Center for Biomedical Imaging, Geneva, Switzerland, 3Medical University of Vienna, Vienna, Austria, 4Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 5Massachusetts General Hospital, Boston, MA, United States, 6Brigham and Women Hospital, Boston, MA, United States
Synopsis
Metabolic alterations specific to glioma can be imaged by MR spectroscopic imaging (MRSI). Adiabatic spin-echo (ASE) MRSI enables spectral editing for specific metabolites with uniform excitation over whole-brain but needs long TR at 7T due to specific absorption rate which results in long acquisition times for high spatial resolution. Acceleration of ASE can be obtained with non-cartesian compressed sense MRSI (ECCENTRIC) acquisition. Here we evaluate the performance of ASE-ECCENTRIC metabolic imaging in glioma patients. We expect that enhanced spectral sensitivity, specificity and high spatial resolution of ASE-ECCENTRIC will improve diagnosis, prognostication, and treatment response monitoring in glioma patients.
Introduction
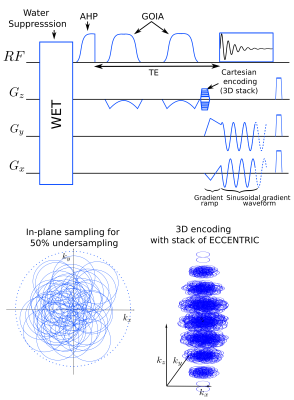
Metabolic alterations can be observed with MR spectroscopic imaging (MRSI) in brain of glioma patients and enables tumor typing and grading, but clinical acquisition are long and performed with low resolution1. The delineation of tumors and the treatment response could be improved by metabolic images with increased spatial resolution2. Novel MRSI acquisition strategies are highly needed as conventional MRSI techniques are particularly time consuming to acquire at high-resolution3. In this study, we combined an adiabatic spin-echo sequence with the sampling strategy of ECCENTRIC, a novel non-cartesian and random k-space trajectories for MRSI at 7Tesla. Acquisitions were performed on glioma patients to assess the capability of imaging metabolic alterations.The pupose of ECCENTRIC, (ECcentric Circle ENcoding TRajectorIes for Compressed-sensing) is to enable highly accelerated high-resolution MRSI for use in clinics4. ECCENTRIC trajectory consists of circles trajectories that are randomly placed in the k-space and permit to circumvent the need for temporal interleaves. Also, the random positioning of the circles combined with specific low-rank and constrained model reconstruction permits to avoid coherent artefact in the image space when random undersampling (compressed sensing) is performed. This strategy permits an acceleration of magnitude 100 in comparison to standard Cartesian MRSI encoding4.
Methods
MR acquisition:The adiabatic spin-echo ECCENTRIC sequence was developed and implemented on a 7T MRI (Terra, Siemens, Erlangen, Germany) with a 32Rx/1Tx NOVA head coil. The ASE excitation used an adiabatic half-passage AHP-HS8 pulse (4ms×5kHz) and two successive GOIA-W16,4 (5ms×20kHz) slab selective refocusing pulses5. The acquisition parameters included: 78 ms echo time, 1400 ms repetition-time was 1400ms, field-of-view (A/P-R/L-H/F) 220×220×105 mm3, 44×44×21 matrix with 5 mm isotropic spatial resolution. The ECCENTRIC circle diameter was set to 2/3|kmax| that allowed 2326Hz spectral bandwidth with no temporal interleaving. Acceleration factor 2 by random undersampling resulted in 15min:50s acquisition time. MRSI data were reconstructed with a low-rank compressed-sense model6 and metabolic maps were created after spectral fitting with LCModel7. A water reference acquisition was performed with the same parameters but a rosette trajectory and a smaller matrix size 18×18×13 (12x12x8mm³) in 2min:50s. Anatomical images were acquired with MP2RAGE at 1 mm isotropic resolution and FLAIR at 1.0×1.0×3.0 mm³ resolution for structural anatomy.
Glioma patients demographics: 6 glioma patients (2 Glioblastoma, 1 anaplastic astrocytoma, 2 oligodendroglioma, 1 anaplastic oligodendroglioma; 6 mutant IDH; 4 19/19q codeleted; age 28-62; 3M/3F) were scanned with informed consent.
Metabolic imaging quantification: A region-of-interest (ROI) was created based on the FLAIR signal hyperintensity in the tumor region and coregistered to the metabolite 3D volume. We created a contralateral ROI to enable a comparisaon with healthy tissue. All the MRSI voxels in the ROIs with LCModel Cramer-Rao Lower Bound (CRLB) < 30%, linewidth < 0.1 ppm and SNR >3 were analysed and the mean and standard deviation was computed for each tumor and contralateral region. Significance of the observed differences were assessed by paired t-test.
Results
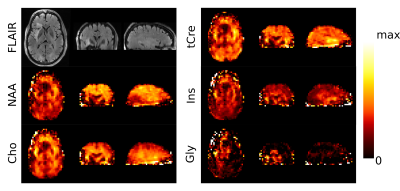
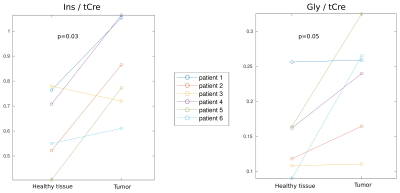
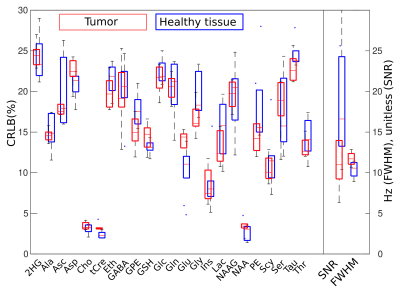
Metabolic imaging of a glioma patient performed with ASE-ECCENTRIC is shown in Figure 2. The Ins, Cho and Gly maps show the most pronunced contrast for this acquisition at TE78 and 5mm isotropic resolution. In Figure 3, the quantitative analysis confirms this point with a significant increase of Ins/tCre and Gly/tCre in tumors, measured across all patients. Figure 4 illustrates the values of the quality parameters in the lesion. No clear systematic increase in CRLB could be observed but SNR values was lower in tumor. This is probably caused by the drop of NAA signal.Discussion
In this work we assessed the performance of ASE-ECCENTRIC for metabolic imaging in glioma patients. The 3D MRSI acquisition reconstructed with a constrained model, produced metabolite maps with high SNR values (>10) and with 5mm isotropic resolution. Further work is required to fully assess the potential of ASE-ECCENTRIC on gliomas but these preliminary results revealed a promising sensitivity and specificity to the glioma pathology.Acknowledgements
No acknowledgement found.References
1. Maudsley, A.A., et al., Advanced magnetic resonance spectroscopic neuroimaging: Experts' consensus recommendations. NMR Biomed, 2020: p. e4309.
2. Bai, J., J. Varghese, and R. Jain, Adult Glioma WHO Classification Update, Genomics, and Imaging: What the Radiologists Need to Know. Top Magn Reson Imaging, 2020. 29(2): p. 71-82.
3. Oz, G., et al., Clinical proton MR spectroscopy in central nervous system disorders. Radiology., 2014. 270(3): p. 658-79.
4. Klauser, A., et al. ECcentric Circle ENcoding TRajectorIes for Compressed-sensing (ECCENTRIC): A fully random non-Cartesian sparse Fourier domain sampling for MRSI at 7 Tesla. ISMRM annual meeting 2021, # 0835 .
5. Esmaeili, M., et al., An integrated RF-receive/B(0)-shim array coil boosts performance of whole-brain MR spectroscopic imaging at 7 T. Sci Rep, 2020. 10(1): p. 15029-15046.
6. Klauser, A., et al., Achieving high-resolution (1)H-MRSI of the human brain with compressed-sensing and low-rank reconstruction at 7 Tesla. J Magn Reson, 2021. 331: p. 107048.
7. Provencher, S.W., Estimation of Metabolite Concentrations from Localized in-Vivo Proton Nmr-Spectra. Magnetic Resonance in Medicine, 1993. 30(6): p. 672-679.
Figures