4792
AVA Diagnostics - multimodality computer assisted aneurysm management tool1Laboratory of Imaging Technologies, Faculty of Electrical Engineering, University of Ljubljana, Ljubljana, Slovenia, 2Division of Interventional Neuroradiology, Department of Radiological Sciences, Ronald Reagan UCLA Medical Center, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States
Synopsis
Management of (un)ruptured intracranial aneurysms (IAs) through early detection and continuous rupture risk assessment is becoming an integral part of IA treatment. This study showcases the capabilities of AVA Diagnostics tool and its IA workflow including automatic vessel segmentation, detection and isolation, morphologic quantification, future growth prediction and follow-up morphologic analysis. Each of the tools was successfully validated on multimodal MRA, CTA and 3D-DSA scans. The AVA Diagnostics thus aims to simplify the management of IAs through extensive use of computer-assisted image analysis and analytics tools, and enable their application in large-scale studies and in regular clinical workflow.
Introduction
Intracranial aneurysms (IAs) are a common cerebrovascular pathology characterized as abnormal bulges forming on the intracranial vessel wall. Most often IAs are located near the circle of Willis (approximately 85 %)1, but also near vessel bifurcations. Despite high prevalence (2-8% of general population) the majority of IAs do not rupture throughout a patient's lifetime. In the unlikely case of rupture, however, the bleeding from IA may cause hemorrhagic stroke with 50% fatality rate, whereas 66% of survivors suffer from permanent neurological deficits.The IAs are generally clinically silent, but are increasingly more often accidentally discovered before rupture, during regular screening. Since traditional surgical treatments are always associated with a certain risk of complications, i.e. the ISUIA trial showed a morbidity and mortality rate of 12.6% and 10.1% for surgical clipping and endovascular therapy 3, which also progressively increases with age 4,5,, they often do not outweigh the risk of rupture. Management of (un)ruptured aneurysms through early detection and continuous rupture risk assessment is thus becoming an integral part of aneurysm treatment.
In this work we present the validation of AVA Diagnostics, a comprehensive toolset enabling computer-assisted vessel extraction, highly sensitive IA detection, isolation and morphologic analysis, longitudinal monitoring, and rupture risk prediction, all based on the deep learning assisted analysis of CTA, MRA or DSA scans.
Methods
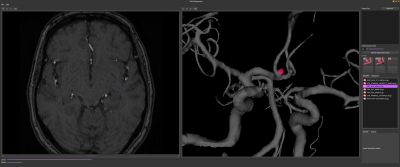
AVA Diagnostics is a standalone tool for medical image visualization, computer-assisted image analysis and image-based analytics. Here we present a workflow dedicated to IA management. The workflow initiates with automated vessel segmentation based on spatially distributed and cascased U-Net models 6,7 trained and validated for each angiographic modality. The obtained segmentation is then transformed into trigonometric mesh using Marching 8 with surface smoothing and completion as post-processing. Subsequent analyses utilized the trigonometric mesh in combination with deep learning based PointNet++ 9 (PN++) model.For IA detection the mesh was subdivided into overlapping patches, which were fed into pretrained PN++ to label likely IA locations. The patch labels were aggregated into original mesh via majority voting to highlight potential IA locations (Figure 1). For each visually confirmed IA its neck curve was extracted using an auto-context based PN++10. The neck curve isolated the IA from parent vessels and the obtained part served to compute rupture risk biomarkers such as size, volume, surface area and nonsphericity 11. Future growth prediction and rupture risk assessment were based on Random Forests with the biomarkers as features, and the PN++ with IA and parent vessel geometry as 12 In case of follow-up scans, the longitudinal morphological development of IA was analyzed using a shape morphing 13.
Validation of the tools was based on 610 MRA, 245 CTA and 57 DSA scans of subjects harbouring one or more IAs, and additional 44 long-term follow-up MRA and CTA scans of unruptured IAs. Each tool was independently validated against relevant expert annotations. Namely, for vessel segmentation we used 570 manually segmented MRA cases of healthy subjects from the IXI dataset 14 and 240 manually segmented CTAs. To validate IA detection and isolation the expert depicted the isolating neck curve at each IA location. The detection was validated 57 3D-DSA, 5 CTA and 106 MRA scans and the IA neck curve isolation on 70 IAs from 3D-DSA, 25 from CTA, and 108 from MRA scans. Models for future growth prediction were trained and tested using cross-validation on the 44 cases with follow-up.
Results
The U-Net models achieved a median Dice overlap value of 0.946, when comparing the obtained and manually segmented MRA scans, and slightly lower value of 0.85 on CTA scans. However, expert visual inspection indicated that the lower Dice values were mainly due to noisy expert annotations. The IA detection showed an overall sensitivity of 0.94 at 0.57 false positives per scan (0.47 on the IXI dataset). Similarly, the IA isolation was highly accurate with a mean distance of 0.351 mm between expert-annotated and automatically extracted neck curve. The Pearson correlation coefficient computed between quantitative IA features extracted manually or based on the automated isolation was >0.97. The best future IA growth prediction model was based on the PN++ and achieved a high sensitivity of 0.96 at 0.63 specificity. The shape morphing based analysis of follow-up scans clearly visualized the morphological changes (Figure 2) and successfully distinguished between growing and stable aneurysms (AUC>0.89).Discussion
The purpose of this study was to showcase the capabilities of the AVA Diagnostics suite, namely the tools for automatic vessel segmentation, IA detection and isolation, morphologic quantification, future growth prediction and follow-up morphologic analysis. Each of the tools was successfully validated on MRA, CTA and 3D-DSA scans. The most unique feature is that, with the exception of vessel segmentation, the tools are based on vessel and IA surface mesh analysis and are thus modality-agnostic, increasing their versatility in real use case scenarios.Conclusion
Suites like the AVA Diagnostics aim to simplify the management of chronic diseases like the IA through extensive use of computer-assisted image analysis and analytics tools, thus enabling their application in large-scale studies and in regular clinical workflow.Acknowledgements
This study was supported by the Slovenian Research Agency (Core Research Grant No. P2-0232 and Research Grants Nos. J2-8173 and J2-2500)
This research is inpart supported by NIH R01HL152270, AHA 18IPA34170130 and UCLA Exploratory Research Grant.
References
Figures