4790
Pulmonary proton density mapping and cardiac 31P MRS suggest an energetic basis for exercise-induced pulmonary congestion in HFpEF1Aarhus University, Aarhus, Denmark, 2OCMR (Oxford Center for Clinical Magnetic Resonance Research), University of Oxford, Oxford, United Kingdom, 3Department of Physics, University of Oxford, Oxford, United Kingdom, 4Department of Imaging Methods, Slovak Academy of Sciences, Bratislava, Slovakia, 5Department of Cardiology, Oxford University Hospitals NHS Trust, Oxford, United Kingdom
Synopsis
We show in 43 patients with 31P MRS, 1H CMR and a novel proton-density mapping sequence that a gradient of myocardial energetic impairment exists across a spectrum of HFpEF phenotypes of increasing clinical severity and worsening diastolic function. A greater degree of myocardial energetic deficit is linked to impaired LV systolic and diastolic functional reserve, to altered RV reserve and RV-PA coupling, and to exercise-induced pulmonary congestion assessed using novel proton density magnetic resonance imaging. A subgroup of HFpEF patients demonstrate transient pulmonary congestion during exercise which can be non-invasively assessed using exercise CMR and proton density mapping.
Introduction
Heart failure with preserved ejection fraction (HFpEF) represents approximately 50% of heart failure cases, providing a highly prevalent morbidity burden worldwide.[1] Transient pulmonary congestion during exercise is an important determinant of reduced exercise capacity in HFpEF patients, who are significantly limited in day-to-day activities by their condition. We sought to determine if an abnormal cardiac energetic state underpins this process through combined cardiac 31P MRS, retrospectively-gated exercise-tolerant proton CMR imaging, and a novel proton density mapping sequence to quantify pulmonary congestion. We wished also to assess the right heart, and atria, and the functional reserve of these patients during exercise stress.Methods
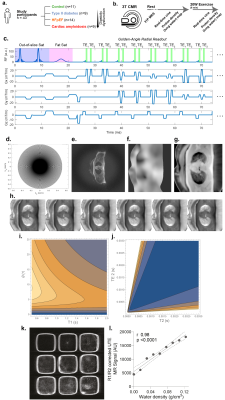
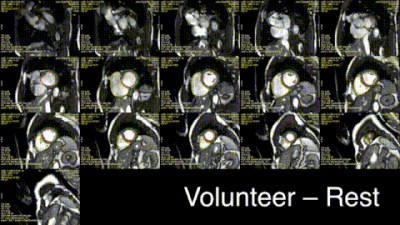
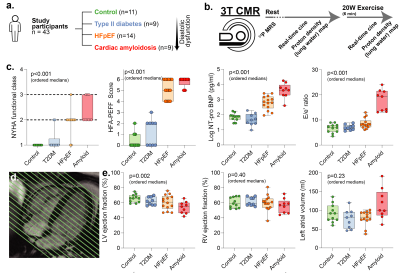
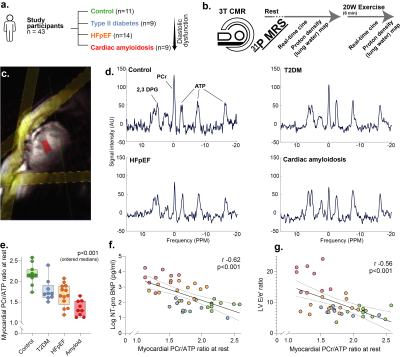
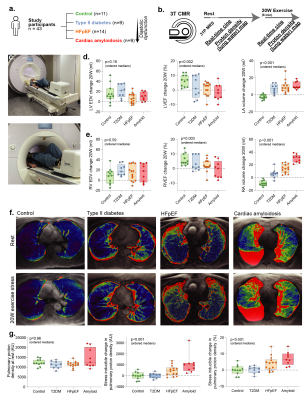
We recruited participants across the spectrum of diastolic dysfunction (controls n=11, type 2 diabetes (T2DM) n=9, HFpEF n=14), with 99mTc-DPD +ve cardiac amyloidosis patients (n=9) as positive controls. After measuring cardiac phosphocreatine/ATP ratio via a UTE-CSI MRSI sequence (Siemens Tim Trio 3T) with a nominal voxel size of 11.25 ml in a $$$16\times8\times8$$$ matrix,[2] we invited participants to undergo real-time CINE-MRI at rest and during mild exercise (20 W) with an MRI-compatible stepping ergometer (Cardio Step, Ergospect-GmbH). This sequence was a cartesian retrospectively-gated, compressed sensing four-fold accelerated 2D bSSFP/BEAT-2CV-based imaging sequence reconstructed in Gadgetron, as described previously.[3]To image pulmonary congestion, we developed a rapid compressed-sensing proton-density mapping radial UTE sequence to estimate pulmonary density before/after exercise. We note that, at low flip angle, the spoiled gradient-echo signal equation is approximately linear in proton density, flip angle, and $$$e^{-T_E/T_{2}^{*}}$$$. We hence estimated $$$T_{2}^{*}$$$ with dual-echo TE=70 µs/1.8 ms, optimised via out-of-slice saturation and fat-saturation pulses. A single slice was acquired continuously for approximately 60 s with a 5º flip-angle, a 6 ms TR, and a 10 mm slab ($$$512\times12=6144$$$ radial golden-angle spokes; $$$384\,\text{mm}^{2}$$$ FOV). The reconstruction was: (1) gradient ringdown estimation and NUFFT via BART[4], (2) coil sensitivity map estimation (ESPiRIT); (3) the reconstruction of multiple single-cardiac-phase images by a sliding window approach, to minimise exercise-induced inhomogeneity; before (4) reconstruction via ADMM-PICS ($$$\beta=2,\,\rho=0.005,\,\lambda=0.001$$$) at twice the acquisition FOV, so that motion artefacts were outside the body. The resulting set of ~6 same-phase images were then summed.
After multi-echo reconstruction, a point estimate of T2* was computed from $$$T_2^{\ast}={\Delta}\text{TE}/\ln(S(\text{TE}_1)/S(\text{TE}_2))$$$; and image intensity scaled according to the gradient-echo signal equation assuming a constant T1 of 1s, as it was infeasible to estimate T1 in the six minutes that these patients can maintain 20 W exertion. This does not represent a significant limitation, as with a 5º FA the GRE signal equation becomes $$$S\approx\rho_{^{1}\text{H}}\left(\theta+\mathcal{O}(\theta^3\cdot\,f(\text{TR}/T_1)\right)e^{-\text{TE}/T_{2}^{*}}$$$ and thus the error due to T1 is quantitatively small (Fig.1.) with:[5]$$\left|\frac{\delta\,T_{2}^{*}/T_{2}^{*}}{\delta{}S/S}\right|=\frac{\sqrt{e^{2\text{TE}_1/T_{2}^{*}}+e^{2\text{TE}_2/T_{2}^{*}}}}{(\text{TE}_2-\text{TE}_1)/T_{2}^{*}}.$$
The sequence was validated in a water-loaded sponge phantom with a physiological pore size prior to human use (Fig.1).
Results
Excellent, artefact-free CMR data were obtained during exercise (Fig.2) and quantified with Cmr42. We found (Figs.3-5) a gradient of reduced myocardial PCr/ATP across the spectrum of diastolic dysunfction and cardiac amyloidosis (median$$$^{+\delta_+}_{-\delta_-}$$$ to cover a 95%-CI: control $$$\text{PCr}/\text{ATP}=2.15^{+0.14}_{-0.06}$$$, T2DM $$$=1.71^{+0.20}_{-0.10}$$$, HFpEF $$$=1.66^{+0.23}_{-0.22}$$$, cardiac amyloidosis $$$=1.30^{+0.23}_{-0.14}$$$, p<0.001). Along with these changes, pulmonary proton density mapping revealed transient pulmonary congestion following 20 W exercise in patients with HFpEF ($$$+4.4_{-3.9}^{+2.0}\,\%$$$, p=0.002) and cardiac amyloidosis ($$$+6.4_{-3.1}^{+3.6}\,\%$$$, p=0.004), which was not seen in healthy controls ($$$-0.1^{+2.2}_{-1.8}\,\%$$$, p=0.89) or T2DM patients without HFpEF ($$$+0.8^{+1.1}_{-2.5}\,\%$$$, p=0.82). Importantly, the development of exercise-induced pulmonary congestion was associated with lower PCr/ATP (Pearson's $$$r=-0.43$$$, p=0.004). During 20 W exercise, lower diastolic filling rates were seen in along the spectrum, together with adverse changes in other cardiac parameters, such as diastolic filling rates and consistent with impaired diastolic function. There were no differences in CMR-derived LV/RV end-diastolic volumes at rest, but there was an increase in median LV mass across ordered groups. Relevant blood biochemical parameters, such as NT-proBNP were similarly concordant.Discussion
Known aetiological risk factors for HFpEF including aging, T2DM, obesity and hypertension, are all known to have links to mitochondrial deficits. Whilst the biomolecular basis of these deficits may vary, we have demonstrated a symptomatic link to impaired metabolism (reduced PCr/ATP) consistent with the clinical phenotype. Although patients in this study had a preserved LV ejection fraction at rest, there was a markedly abnormal left heart response to low-intensity exercise which was characterised by reduced peak LV filling rate, blunted LV contractile reserve, and left atrial dilatation. The right heart response was similarly abnormal, with RV-PA uncoupling, evidenced by impaired RV functional reserve and RA dilatation. These results are consistent with increased LA pressure, and elevated systemic venous pressure due to stagnation; they individually correlated with PCr/ATP. Consistent with a pressure-overload hypothesis, a subgroup of HFpEF patients demonstrate transient pulmonary congestion during exercise which can be non-invasively assessed using exercise CMR and proton density mapping. We therefore present a non-invasive characterisation of pulmonary congestion in HFpEF, and hint at its metabolic basis.Conclusion
These results suggest that a gradient of energetic deficit exists across the spectrum of HFpEF, and even at very modest energetic workloads of 20W a substantially abnormal exercise response is elucidated. We detected pulmonary congestion in HFpEF/amyloid patients, correlated with a lower PCr/ATP ratio, suggesting an energetic basis in this prevalent and morbid disease. Metabolic alteration may thus be a promising strategy in HFpEF.Acknowledgements
This study was principally funded by a British Heart
Foundation Intermediate Clinical Research Fellowship FS/16/70/32157 (to Dr
Rider). Dr Burrage acknowledges support from a British Heart Foundation
Clinical Research Training Fellowship (FS/19/65/34692). Dr Valkovič is
supported by the Sir Henry Dale Fellowship, jointly funded by the Royal Society
and the Wellcome Trust (221805/Z/20/Z), and also acknowledges support of the
Slovak Grant Agencies VEGA (2/0003/20) and APVV (#19-0032). Dr Miller was
supported by a Novo Nordisk Postdoctoral Fellowship run in conjunction with the
University of Oxford. SN, AL and OR acknowledge support from the British Heart
Foundation Oxford Centre of Research Excellence. AL, MKB and SN acknowledge
support from the National Institute for Health Research (NIHR) Oxford
Biomedical Research Centre. The authors acknowledge Adrienne G Siu, Iulius Dragonu, Luca Biasiolli, and Matthew D Robson for providing the original UTE sequence upon which the lung water sequence was developed.
References
1. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nature Reviews Cardiology. 2020;17:559–573.
2. Tyler DJ, Emmanuel Y, Cochlin LE, Hudsmith LE, Holloway CJ, Neubauer S, Clarke K, Robson MD. Reproducibility of 31P cardiac magnetic resonance spectroscopy at 3 T. NMR in Biomedicine. 2009;22:405–413.
3. Xue, H., Kellman, P., LaRocca, G., Arai, A. E. & Hansen, M. S. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J. Cardiovasc. Magn. Reson. 15, 102 (2013).
4. Uecker, M., Tamir, J. I., Ong, F. & Lustig, M. The BART Toolbox for Computational Magnetic Resonance Imaging. in Proc. Intl. Soc. Mag. reson. Med. 24 (2016).
5. Gai, N. D., Malayeri, A. A. & Bluemke, D. A. Three-dimensional T1 and T2* mapping of human lung parenchyma using interleaved saturation recovery with dual echo ultrashort echo time imaging (ITSR-DUTE). J. Magn. Reson. Imaging 45, 1097–1104 (2017).
Figures